Maxizyme-mediated specific inhibition on mutant-type p53 in vitro
- PMID: 12854166
- PMCID: PMC4615507
- DOI: 10.3748/wjg.v9.i7.1571
Maxizyme-mediated specific inhibition on mutant-type p53 in vitro
Abstract
Aim: To evaluate the specific inhibition of maxizyme directing against mutant-type p53 gene (mtp53) at codon 249 in exon 7 (AGG-AGT) in vitro.
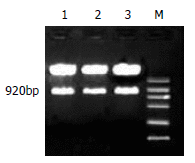
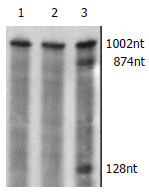
Methods: Two different monomers of anti-mtp53 maxizyme (maxizyme right MzR, maxizyme left MzL) and control mutant maxizyme (G(5)-A(5)) were designed by computer and cloned into vector pBSKU6 (pBSKU6MzR, pBSKU6MzL). After being sequenced, the restrictive endonuclease site in pBSKU6MzR was changed by PCR and then U6MzR was inserted into pBSKU6MzL, the recombinant vector was named pU6Mz and pU6asMz (mutant maxizyme). Mtp53 and wild-type p53 (wtp53) gene fragments were cloned into pGEM-T vector under the T7 promoter control. The (32)p-labeled mtp53 transcript was the target mRNA. Cold maxizyme transcripts were incubated with (32)p-labeled target RNA in vitro and radioautographed after denaturing polyacrylamide gel electrophoresis.
Results: In cell-free systems, pU6Mz showed a specific cleavage activity against target mRNA at 37 degrees and 25 mM MgCL(2). The cleavage efficiency of pU6Mz was 42 %, while pU6asMz had no inhibitory effect. Wtp53 was not cleaved by pU6Mz either.
Conclusion: pU6Mz had a specific catalytic activity against mtp53 in cell-free system. These lay a good foundation for studying the effects of anti-mtp53 maxizyme in HCC cell lines. The results suggest that maxizyme may be a promising alternative approach for treating hepatocellular carcinoma containing mtp53.
Figures



Similar articles
-
[Inhibition of mutant-type p53 by a chimeric U6 maxizyme in hepatocellular carcinoma cell lines].Zhonghua Gan Zang Bing Za Zhi. 2005 Oct;13(10):759-62. Zhonghua Gan Zang Bing Za Zhi. 2005. PMID: 16248949 Chinese.
-
[Inhibition of mutant p53 in hepatocellular carcinoma cells by hammerhead ribozyme].Zhonghua Gan Zang Bing Za Zhi. 2003 Dec;11(12):722-4. Zhonghua Gan Zang Bing Za Zhi. 2003. PMID: 14697131 Chinese.
-
Cleavage of an inaccessible site by the maxizyme with two independent binding arms: an alternative approach to the recruitment of RNA helicases.J Biochem. 2002 Jul;132(1):149-55. doi: 10.1093/oxfordjournals.jbchem.a003193. J Biochem. 2002. PMID: 12097172
-
[Target therapy for CML--applying maxizyme].Nihon Rinsho. 2001 Dec;59(12):2439-44. Nihon Rinsho. 2001. PMID: 11766353 Review. Japanese.
-
Role of Reactivating Mutant p53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer.Onco Targets Ther. 2022 Jan 8;15:23-30. doi: 10.2147/OTT.S342292. eCollection 2022. Onco Targets Ther. 2022. PMID: 35035222 Free PMC article. Review.
Cited by
-
Functional repair of p53 mutation in colorectal cancer cells using trans-splicing.Oncotarget. 2015 Feb 10;6(4):2034-45. doi: 10.18632/oncotarget.2988. Oncotarget. 2015. PMID: 25576916 Free PMC article.
References
-
- Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Huaren Xiaohua Zazhi. 1998;6:1013–1016.
-
- Zhai SH, Liu JB, Liu YM, Zhang LL, Du ZH. Expression of HBsAg, HCV-Ag and AFP in liver cirrhosis and hepatocarcinoma. Shijie. Huaren Xiaohua Zazhi. 2000;8:524–527.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

