Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media
- PMID: 12855765
- PMCID: PMC166410
- DOI: 10.1073/pnas.1432026100
Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media
Abstract
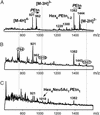
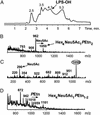
Otitis media, a common and often recurrent bacterial infection of childhood, is a major reason for physician visits and the prescription of antimicrobials. Haemophilus influenzae is the cause of approximately 20% of episodes of bacterial otitis media, but most strains lack the capsule, a factor known to play a critical role in the virulence of strains causing invasive H. influenzae disease. Here we show that in capsule-deficient (nontypeable) strains, sialic acid, a terminal residue of the core sugars of H. influenzae lipopolysaccharide (LPS), is a critical virulence factor in the pathogenesis of experimental otitis media in chinchillas. We used five epidemiologically distinct H. influenzae isolates, representative of the genetic diversity of strains causing otitis media, to inoculate the middle ear of chinchillas. All animals developed acute bacterial otitis media that persisted for up to 3 wk, whereas isogenic sialic acid-deficient mutants (disrupted sialyltransferase or CMP-acetylneuraminic acid synthetase genes) were profoundly attenuated. MS analysis indicated that WT bacteria used to inoculate animals lacked any sialylated LPS glycoforms. In contrast, LPS of ex vivo organisms recovered from chinchilla middle ear exudates was sialylated. We conclude that sialylated LPS glycoforms play a key role in pathogenicity of nontypeable H. influenzae and depend on scavenging the essential precursors from the host during the infection.
Figures



References
-
- Teele, D. W., Klein, J. O., Rosner, B., Bratton, L., Fisch, G. R., Mathieu, O. R., Porter, P. J., Starobin, S. G., Tarlin, L. D. & Younes, R. P. (1983) J. Am. Med. Assoc. 249, 1026–1029. - PubMed
-
- Klein, J. O. (2000) Vaccine 19, Suppl. 1, S2–S8. - PubMed
-
- Kilpi, T., Herva, E., Kaijalainen, T., Syrjanen, R. & Takala, A. K. (2001) Pediatr. Infect. Dis. J. 20, 654–662. - PubMed
-
- Bernstein, J. M., Faden, H. S., Loos, B. G., Murphy, T. F. & Ogra, P. L. (1992) Int. J. Pediatr. Otorhinolaryngol. 23, 1–13. - PubMed
-
- Shurin, P. A., Pelton, S. I., Tager, I. B. & Kasper, D. L. (1980) J. Pediatr. 97, 364–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

