Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180
- PMID: 12856000
- PMCID: PMC1326338
- DOI: 10.1038/sj.embor.embor898
Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180
Abstract
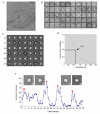
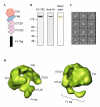
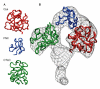
Endo180, also known as the urokinase plasminogen activator receptor (uPAR)-associated protein (uPARAP), is one of the four members of the mannose receptor family, and is implicated in extracellular-matrix remodelling through its interactions with collagens, sugars and uPAR. The extracellular portion of Endo180 contains an amino-terminal cysteine-rich domain, a single fibronectin type II domain and eight C-type lectin-like domains. We have purified a soluble version of Endo180 and analysed it by single-particle electron microscopy to obtain a three-dimensional structure of the N-terminal part of the protein at a resolution of 17 A and reveal, for the first time, the interactions between non-adjacent domains in the mannose receptor family. We show that for Endo180, the cysteine-rich domain contacts the second C-type lectin-like domain, thus providing structural insight into how modulation of its several ligand interactions may regulate Endo180 receptor function.
Figures
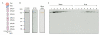

References
-
- Ancian P., Lambeau G. & Lazdunski M. ( 1995) Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry, 34, 13146–13151. - PubMed
-
- Behrendt N., Jensen O.N., Engelholm L.H., Mortz E., Mann M. & Danø K. ( 2000) A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem., 275, 1993–2002. - PubMed
-
- East L. & Isacke C.M. ( 2002) The mannose receptor family. Biochim. Biophys. Acta, 1572, 364–386. - PubMed
-
- East L., Rushton S., Taylor M.E. & Isacke C.M. ( 2002) Characterization of sugar binding by the mannose receptor family member, Endo180. J. Biol. Chem., 277, 50469–50475. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

