Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock
- PMID: 12857774
- PMCID: PMC164216
- DOI: 10.1128/CMR.16.3.379-414.2003
Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock
Abstract
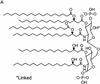
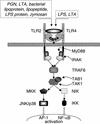
Bacterial sepsis and septic shock result from the overproduction of inflammatory mediators as a consequence of the interaction of the immune system with bacteria and bacterial wall constituents in the body. Bacterial cell wall constituents such as lipopolysaccharide, peptidoglycans, and lipoteichoic acid are particularly responsible for the deleterious effects of bacteria. These constituents interact in the body with a large number of proteins and receptors, and this interaction determines the eventual inflammatory effect of the compounds. Within the circulation bacterial constituents interact with proteins such as plasma lipoproteins and lipopolysaccharide binding protein. The interaction of the bacterial constituents with receptors on the surface of mononuclear cells is mainly responsible for the induction of proinflammatory mediators by the bacterial constituents. The role of individual receptors such as the toll-like receptors and CD14 in the induction of proinflammatory cytokines and adhesion molecules is discussed in detail. In addition, the roles of a number of other receptors that bind bacterial compounds such as scavenger receptors and their modulating role in inflammation are described. Finally, the therapies for the treatment of bacterial sepsis and septic shock are discussed in relation to the action of the aforementioned receptors and proteins.
Figures


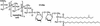



References
-
- Abraham, E. 1999. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25:556-566. - PubMed
-
- Acton, S., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518-520. - PubMed
-
- Agellon, L. B., E. M. Quinet, T. G. Gillette, D. T. Drayna, M. L. Brown, and A. R. Tall. 1990. Organization of the human cholesteryl ester transfer protein gene. Biochemistry 29:1372-1376. - PubMed
-
- Akira, S. 2000. Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28:551-556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

