Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes
- PMID: 12857780
- PMCID: PMC164223
- DOI: 10.1128/CMR.16.3.517-533.2003
Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes
Abstract
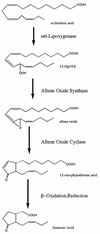
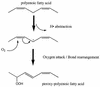
Oxylipins are oxygenated metabolites of fatty acids. Eicosanoids are a subset of oxylipins and include the prostaglandins and leukotrienes, which are potent regulators of host immune responses. Host cells are one source of eicosanoids and oxylipins during infection; however, another potential source of eicosanoids is the pathogen itself. A broad range of pathogenic fungi, protozoa, and helminths produce eicosanoids and other oxylipins by novel synthesis pathways. Why do these organisms produce oxylipins? Accumulating data suggest that phase change and differentiation in these organisms are controlled by oxylipins, including prostaglandins and lipoxygenase products. The precise role of pathogen-derived eicosanoids in pathogenesis remains to be determined, but the potential link between pathogen eicosanoids and the development of TH2 responses in the host is intriguing. Mammalian prostaglandins and leukotrienes have been studied extensively, and these molecules can modulate Th1 versus Th2 immune responses, chemokine production, phagocytosis, lymphocyte proliferation, and leukocyte chemotaxis. Thus, eicosanoids and oxylipins (host or microbe) may be mediators of a direct host-pathogen "cross-talk" that promotes chronic infection and hypersensitivity disease, common features of infection by eukaryotic pathogens.
Figures





Similar articles
-
Molecular basis for prostaglandin production in hosts and parasites.Trends Parasitol. 2007 Jul;23(7):325-31. doi: 10.1016/j.pt.2007.05.005. Epub 2007 May 24. Trends Parasitol. 2007. PMID: 17531535 Review.
-
Production of cross-kingdom oxylipins by pathogenic fungi: An update on their role in development and pathogenicity.J Microbiol. 2016 Mar;54(3):254-64. doi: 10.1007/s12275-016-5620-z. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920885 Free PMC article. Review.
-
Co-opting oxylipin signals in microbial disease.Cell Microbiol. 2019 Jun;21(6):e13025. doi: 10.1111/cmi.13025. Cell Microbiol. 2019. PMID: 30866138 Free PMC article. Review.
-
The role of oxylipins in NSAID-exacerbated respiratory disease (N-ERD).Adv Pharmacol. 2023;97:423-444. doi: 10.1016/bs.apha.2022.12.002. Epub 2023 Jan 10. Adv Pharmacol. 2023. PMID: 37236766 Free PMC article.
-
Synthesis and function of fatty acids and oxylipins, with a focus on Caenorhabditis elegans.Prostaglandins Other Lipid Mediat. 2020 Jun;148:106426. doi: 10.1016/j.prostaglandins.2020.106426. Epub 2020 Feb 4. Prostaglandins Other Lipid Mediat. 2020. PMID: 32032704 Review.
Cited by
-
Density-Dependent Prophylaxis in Freshwater Snails Driven by Oxylipin Chemical Cues.Front Immunol. 2022 Jan 31;13:826500. doi: 10.3389/fimmu.2022.826500. eCollection 2022. Front Immunol. 2022. PMID: 35173735 Free PMC article.
-
Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids.Biochim Biophys Acta. 2015 Apr;1851(4):456-68. doi: 10.1016/j.bbalip.2014.11.012. Epub 2014 Dec 5. Biochim Biophys Acta. 2015. PMID: 25486530 Free PMC article. Review.
-
Targeting host-specific metabolic pathways-opportunities and challenges for anti-infective therapy.Front Mol Biosci. 2024 Feb 22;11:1338567. doi: 10.3389/fmolb.2024.1338567. eCollection 2024. Front Mol Biosci. 2024. PMID: 38455763 Free PMC article. Review.
-
Inactivation of the phospholipase B gene PLB5 in wild-type Candida albicans reduces cell-associated phospholipase A2 activity and attenuates virulence.Int J Med Microbiol. 2006 Oct;296(6):405-20. doi: 10.1016/j.ijmm.2006.03.003. Epub 2006 Jun 6. Int J Med Microbiol. 2006. PMID: 16759910 Free PMC article.
-
Directed Non-targeted Mass Spectrometry and Chemical Networking for Discovery of Eicosanoids and Related Oxylipins.Cell Chem Biol. 2019 Mar 21;26(3):433-442.e4. doi: 10.1016/j.chembiol.2018.11.015. Epub 2019 Jan 17. Cell Chem Biol. 2019. PMID: 30661990 Free PMC article.
References
-
- Abdel Baset, H., G. P. O'Neill, and A. W. Ford-Hutchinson. 1995. Characterization of arachidonic-acid-metabolizing enzymes in adult Schistosoma mansoni. Mol. Biochem. Parasitol. 73:31-41. - PubMed
-
- Ali, S. F., A. Joachim, and A. Daugschies. 1999. Eicosanoid production by adult Fasciola hepatica and plasma eicosanoid patterns during fasciolosis in sheep. Int. J. Parasitol. 29:743-748. - PubMed
-
- Aliberti, J., and A. Sher. 2001. Positive and negative regulation of pathogen induced dendritic cell function by G-protein coupled receptors. Mol. Immunol. 38:891-893. - PubMed
-
- Aliberti, J., and A. Sher. 2002. Role of G-protein-coupled signaling in the induction and regulation of dendritic cell function by Toxoplasma gondii. Microbes Infect. 4:991-997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

