Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress
- PMID: 12857805
- PMCID: PMC167063
- DOI: 10.1104/pp.102.017707
Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress
Abstract
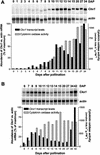
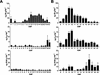
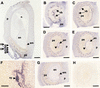
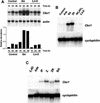
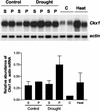
Cytokinins are hormones that play an essential role in plant growth and development. The irreversible degradation of cytokinins, catalyzed by cytokinin oxidase, is an important mechanism by which plants modulate their cytokinin levels. Cytokinin oxidase has been well characterized biochemically, but its regulation at the molecular level is not well understood. We isolated a cytokinin oxidase open reading frame from maize (Zea mays), called Ckx1, and we used it as a probe in northern and in situ hybridization experiments. We found that the gene is expressed in a developmental manner in the kernel, which correlates with cytokinin levels and cytokinin oxidase activity. In situ hybridization with Ckx1 and transgenic expression of a transcriptional fusion of the Ckx1 promoter to the Escherichia coli beta-glucuronidase reporter gene revealed that the gene is expressed in the vascular bundles of kernels, seedling roots, and coleoptiles. We show that Ckx1 gene expression is inducible in various organs by synthetic and natural cytokinins. Ckx1 is also induced by abscisic acid, which may control cytokinin oxidase expression in the kernel under abiotic stress. We hypothesize that under non-stress conditions, cytokinin oxidase in maize plays a role in controlling growth and development via regulation of cytokinin levels transiting in the xylem. In addition, we suggest that under environmental stress conditions, cytokinin oxidase gene induction by abscisic acid results in aberrant degradation of cytokinins therefore impairing normal development.
Figures





References
-
- Armstrong DJ (1994) Cytokinin oxidase and the regulation of cytokinin degradation. In DWS Mok, MC Mok, eds, Cytokinins, Chemistry, Activity and Function. CRC Press, Boca Raton, FL, pp 139–154
-
- Bertell G, Eliasson L (1992) Cytokinin effects on root growth and possible interactions with ethylene and indol-3-acetic acid. Physiol Plant 84: 255–261
-
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997) The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J 11: 339–345
-
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E (1993) Complementary floral homeoetic phenotypes result from opposite orientation of a transposon at the plena locus of Antirrhinum. Cell 72: 85–95 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

