Phytoremediation of organomercurial compounds via chloroplast genetic engineering
- PMID: 12857816
- PMCID: PMC167074
- DOI: 10.1104/pp.103.020958
Phytoremediation of organomercurial compounds via chloroplast genetic engineering
Abstract
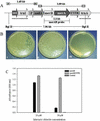
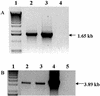
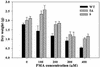
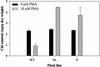
Mercury (Hg), especially in organic form, is a highly toxic pollutant affecting plants, animals, and man. In plants, the primary target of Hg damage is the chloroplast; Hg inhibits electron transport and photosynthesis. In the present study, chloroplast genetic engineering is used for the first time to our knowledge to enhance the capacity of plants for phytoremediation. This was achieved by integrating a native operon containing the merA and merB genes (without any codon modification), which code for mercuric ion reductase (merA) and organomercurial lyase (merB), respectively, into the chloroplast genome in a single transformation event. Stable integration of the merAB operon into the chloroplast genome resulted in high levels of tolerance to the organomercurial compound, phenylmercuric acetate (PMA) when grown in soil containing up to 400 micro M PMA; plant dry weights of the chloroplast transformed lines were significantly higher than those of wild type at 100, 200, and 400 micro M PMA. That the merAB operon was stably integrated into the chloroplast genome was confirmed by polymerase chain reaction and Southern-blot analyses. Northern-blot analyses revealed stable transcripts that were independent of the presence or absence of a 3'-untranslated region downstream of the coding sequence. The merAB dicistron was the more abundant transcript, but less abundant monocistrons were also observed, showing that specific processing occurs between transgenes. The use of chloroplast transformation to enhance Hg phytoremediation is particularly beneficial because it prevents the escape of transgenes via pollen to related weeds or crops and there is no need for codon optimization to improve transgene expression. Chloroplast transformation may also have application to other metals that affect chloroplast function.
Figures



References
-
- Begley TP, Walts AE, Walsh CT (1986) Bacterial organomercurial lyase: overproduction, isolation, and characterization. Biochemistry 25: 7186–7192 - PubMed
-
- Bendich AJ (1987) Why do chloroplasts and mitochondria contain so many copies of their genome? Bioassays 6: 279–282 - PubMed
-
- Bernier M, Popovic R, Carpentier R (1993) Mercury inhibition of photosystem II. FEBS Lett 32: 19–23 - PubMed
-
- Bizily S, Rugh CC, Meagher RB (2000) Phytoremediation of hazardous organomercurials by genetically engineered plants. Nat Biotechnol 18: 213–217 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

