An isoleucine residue within the carboxyl-transferase domain of multidomain acetyl-coenzyme A carboxylase is a major determinant of sensitivity to aryloxyphenoxypropionate but not to cyclohexanedione inhibitors
- PMID: 12857850
- PMCID: PMC167108
- DOI: 10.1104/pp.103.021139
An isoleucine residue within the carboxyl-transferase domain of multidomain acetyl-coenzyme A carboxylase is a major determinant of sensitivity to aryloxyphenoxypropionate but not to cyclohexanedione inhibitors
Abstract
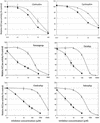
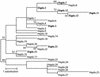
A 3,300-bp DNA fragment encoding the carboxyl-transferase domain of the multidomain, chloroplastic acetyl-coenzyme A carboxylase (ACCase) was sequenced in aryloxyphenoxypropionate (APP)-resistant and -sensitive Alopecurus myosuroides (Huds.). No resistant plant contained an Ile-1,781-Leu substitution, previously shown to confer resistance to APPs and cyclohexanediones (CHDs). Instead, an Ile-2,041-Asn substitution was found in resistant plants. Phylogenetic analysis of the sequences revealed that Asn-2,041 ACCase alleles derived from several distinct origins. Allele-specific polymerase chain reaction associated the presence of Asn-2,041 with seedling resistance to APPs but not to CHDs. ACCase enzyme assays confirmed that Asn-2,041 ACCase activity was moderately resistant to CHDs but highly resistant to APPs. Thus, the Ile-2,041-Asn substitution, which is located outside a domain previously shown to control sensitivity to APPs and CHDs in wheat (Triticum aestivum), is a direct cause of resistance to APPs only. In known multidomain ACCases, the position corresponding to the Ile/Asn-2,041 residue in A. myosuroides is occupied by an Ile or a Val residue. In Lolium rigidum (Gaud.), we found Ile-Asn and Ile-Val substitutions. The Ile-Val change did not confer resistance to the APP clodinafop, whereas the Ile-Asn change did. The position and the particular substitution at this position are of importance for sensitivity to APPs.
Figures

Similar articles
-
An isoleucine/leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors.Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6617-22. doi: 10.1073/pnas.121172798. Epub 2001 May 29. Proc Natl Acad Sci U S A. 2001. PMID: 11381131 Free PMC article.
-
The molecular bases for resistance to acetyl co-enzyme A carboxylase (ACCase) inhibiting herbicides in two target-based resistant biotypes of annual ryegrass (Lolium rigidum).Planta. 2006 Feb;223(3):550-7. doi: 10.1007/s00425-005-0095-x. Epub 2005 Aug 23. Planta. 2006. PMID: 16133206
-
PCR-based detection of resistance to acetyl-CoA carboxylase-inhibiting herbicides in black-grass (Alopecurus myosuroides Huds) and ryegrass (Lolium rigidum gaud).Pest Manag Sci. 2002 May;58(5):474-8. doi: 10.1002/ps.485. Pest Manag Sci. 2002. PMID: 11997974
-
Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: evaluation using clethodim.Plant Physiol. 2007 Oct;145(2):547-58. doi: 10.1104/pp.107.105262. Epub 2007 Aug 24. Plant Physiol. 2007. PMID: 17720757 Free PMC article.
-
Resistance to acetyl-CoA carboxylase-inhibiting herbicides.Pest Manag Sci. 2014 Sep;70(9):1405-17. doi: 10.1002/ps.3790. Epub 2014 May 6. Pest Manag Sci. 2014. PMID: 24700409 Review.
Cited by
-
Resistance determination of the ACCase-inhibiting herbicide of clodinafop propargyl in Avena ludoviciana (Durieu), and study of their interaction using molecular docking and simulation.Mol Biol Rep. 2019 Feb;46(1):415-424. doi: 10.1007/s11033-018-4489-9. Epub 2018 Nov 17. Mol Biol Rep. 2019. PMID: 30448893
-
Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5910-5. doi: 10.1073/pnas.0400891101. Epub 2004 Apr 12. Proc Natl Acad Sci U S A. 2004. PMID: 15079078 Free PMC article.
-
Determination and study on dissipation and residue determination of cyhalofop-butyl and its metabolite using HPLC-MS/MS in a rice ecosystem.Environ Monit Assess. 2014 Oct;186(10):6959-67. doi: 10.1007/s10661-014-3902-7. Epub 2014 Jul 11. Environ Monit Assess. 2014. PMID: 25007772
-
Clarification of pathway-specific inhibition by Fourier transform ion cyclotron resonance/mass spectrometry-based metabolic phenotyping studies.Plant Physiol. 2006 Oct;142(2):398-413. doi: 10.1104/pp.106.080317. Epub 2006 Aug 11. Plant Physiol. 2006. PMID: 16905671 Free PMC article.
-
Basis of selectivity of cyhalofop-butyl in Oryza sativa L.Planta. 2006 Jan;223(2):191-9. doi: 10.1007/s00425-005-0075-1. Epub 2005 Sep 14. Planta. 2006. PMID: 16160841
References
-
- Boutsalis P, Karotam J, Powles SB (1999) Molecular basis of resistance to acetolactate synthase-inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic Sci 55: 507–516
-
- Burton JD, Gronwald JW, Keith RA, Somers DA, Gegenbach BG, Wyse DL (1991) Kinetics of inhibition of acetyl-coenzyme A carboxylase by sethoxydim and haloxyfop. Pestic Biochem Physiol 39: 100–109 - PubMed
-
- Christoffers MJ, Berg ML, Messersmith CG (2002) An isoleucine to leucine mutation in acetyl-CoA carboxylase confers herbicide resistance in wild oat. Genome 45: 1049–1056 - PubMed
-
- Cocker KM, Moss SR, Coleman JOD (1999) Multiple mechanisms of resistance to fenaxoprop-P-ethyl in United Kingdom and other European populations of herbicide-resistant Alopecurus myosuroides (black-grass). Pestic Biochem Physiol 65: 169–180
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

