Myo6 facilitates the translocation of endocytic vesicles from cell peripheries
- PMID: 12857860
- PMCID: PMC165672
- DOI: 10.1091/mbc.e02-11-0767
Myo6 facilitates the translocation of endocytic vesicles from cell peripheries
Abstract
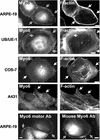
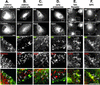
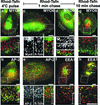
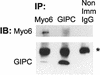
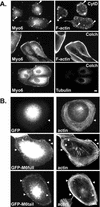
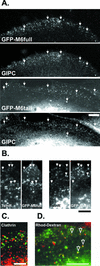
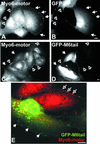
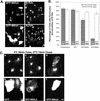
Immunolocalization studies in epithelial cells revealed myo6 was associated with peripherally located vesicles that contained the transferrin receptor. Pulse-chase experiments after transferrin uptake showed that these vesicles were newly uncoated endocytic vesicles and that myo6 was recruited to these vesicles immediately after uncoating. GIPC, a putative myo6 tail binding protein, was also present. Myo6 was not present on early endosomes, suggesting that myo6 has a transient association with endocytic vesicles and is released upon early endosome fusion. Green fluorescent protein (GFP) fused to myo6 as well as the cargo-binding tail (M6tail) alone targeted to the nascent endocytic vesicles. Overexpression of GFP-M6tail had no effect on a variety of organelle markers; however, GFP-M6tail displaced the endogenous myo6 from nascent vesicles and resulted in a significant delay in transferrin uptake. Pulse-chase experiments revealed that transferrin accumulated in uncoated vesicles within the peripheries of transfected cells and that Rab5 was recruited to the surface of these vesicles. Given sufficient time, the transferrin did traffic to the perinuclear sorting endosome. These data suggest that myo6 is an accessory protein required for the efficient transportation of nascent endocytic vesicles from the actin-rich peripheries of epithelial cells, allowing for timely fusion of endocytic vesicles with the early endosome.
Figures
References
-
- Apodaca, G. (2001). Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2, 149–159. - PubMed
-
- Avraham, K.B., Hasson, T., Sobe, T., Balsara, B., Testa, J., Copeland, N.G., and Jenkins, N.A. (1997). Characterization of human unconventional myosin-VI, a gene responsible for deafness in Snell's waltzer mice. Hum. Mol. Genet. 6, 1225–1231. - PubMed
-
- Avraham, K.B., Hasson, T., Steel, K.P., Kingsley, D.M., Russell, L.B., Mooseker, M.S., Copeland, N.G., and Jenkins, N.A. (1995). The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner hair cells. Nat. Genet. 11, 369–374. - PubMed
-
- Biemesderfer, D., Mentone, S.A., Mooseker, M., and Hasson, T. (2002). Expression of myosin-VI within the endocytic pathway in the adult and developing proximal tubule. Am. J. Physiol. 282, F785–F794. - PubMed
-
- Breckler, J., Au, K., Cheng, J., Hasson, T., and Burnside, B. (2000). Novel myosin VI isoform is abundantly expressed in retina. Exp. Eye Res. 70, 121–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

