Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing
- PMID: 12857863
- PMCID: PMC165675
- DOI: 10.1091/mbc.e02-10-0647
Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing
Abstract
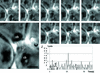
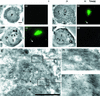
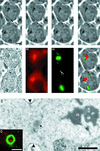
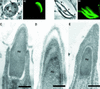
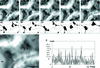
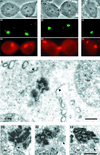
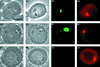
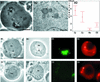
Stable cytoplasmic bridges (or ring canals) connecting the clone of spermatids are assumed to facilitate the sharing of haploid gene products and synchronous development of the cells. We have visualized these cytoplasmic bridges under phase-contrast optics and recorded the sharing of cytoplasmic material between the spermatids by a digital time-lapse imaging system ex vivo. A multitude of small (ca. 0.5 microm) granules were seen to move continuously over the bridges, but only 28% of those entering the bridge were actually transported into other cell. The average speed of the granules decreased significantly during the passage. Immunocytochemistry revealed that some of the shared granules contained haploid cell-specific gene product TRA54. We also demonstrate the novel function for the Golgi complex in acrosome system formation by showing that TRA54 is processed in Golgi complex and is transported into acrosome system of neighboring spermatid. In addition, we propose an intercellular transport function for the male germ cell-specific organelle chromatoid body. This mRNA containing organelle, ca. 1.8 microm in diameter, was demonstrated to go over the cytoplasmic bridge from one spermatid to another. Microtubule inhibitors prevented all organelle movements through the bridges and caused a disintegration of the chromatoid body. This is the first direct demonstration of an organelle traffic through cytoplasmic bridges in mammalian spermatogenesis. Golgi-derived haploid gene products are shared between spermatids, and an active involvement of the chromatoid body in intercellular material transport between round spermatids is proposed.
Figures
Similar articles
-
Assembly of spermatid acrosome depends on microtubule organization during mammalian spermiogenesis.Dev Biol. 2006 May 1;293(1):218-27. doi: 10.1016/j.ydbio.2006.02.001. Epub 2006 Mar 15. Dev Biol. 2006. PMID: 16540102
-
Immunoelectron microscopic study of BASP1 and MARCKS location in the early and late rat spermatids.Acta Histochem. 2012 May;114(3):237-43. doi: 10.1016/j.acthis.2011.06.009. Epub 2011 Jul 20. Acta Histochem. 2012. PMID: 21764106
-
Active movements of the chromatoid body. A possible transport mechanism for haploid gene products.J Cell Biol. 1979 Mar;80(3):621-8. doi: 10.1083/jcb.80.3.621. J Cell Biol. 1979. PMID: 457761 Free PMC article.
-
Transcription in haploid male germ cells.Int Rev Cytol. 2004;237:1-56. doi: 10.1016/S0074-7696(04)37001-4. Int Rev Cytol. 2004. PMID: 15380665 Review.
-
The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head.Arch Histol Cytol. 2004 Nov;67(4):271-84. doi: 10.1679/aohc.67.271. Arch Histol Cytol. 2004. PMID: 15700535 Review.
Cited by
-
Deletion of murine choline dehydrogenase results in diminished sperm motility.FASEB J. 2010 Aug;24(8):2752-61. doi: 10.1096/fj.09-153718. Epub 2010 Apr 6. FASEB J. 2010. PMID: 20371614 Free PMC article.
-
Intercellular Bridge Mediates Ca2+ Signals between Micropatterned Cells via IP3 and Ca2+ Diffusion.Biophys J. 2020 Mar 10;118(5):1196-1204. doi: 10.1016/j.bpj.2020.01.006. Epub 2020 Jan 16. Biophys J. 2020. PMID: 32023438 Free PMC article.
-
Regulation of spermatogenesis by small non-coding RNAs: role of the germ granule.Semin Cell Dev Biol. 2014 May;29:84-92. doi: 10.1016/j.semcdb.2014.04.021. Epub 2014 Apr 19. Semin Cell Dev Biol. 2014. PMID: 24755166 Free PMC article. Review.
-
Soma-to-germline RNA communication.Nat Rev Genet. 2022 Feb;23(2):73-88. doi: 10.1038/s41576-021-00412-1. Epub 2021 Sep 20. Nat Rev Genet. 2022. PMID: 34545247 Review.
-
Human male infertility and its genetic causes.Reprod Med Biol. 2017 Mar 26;16(2):81-88. doi: 10.1002/rmb2.12017. eCollection 2017 Apr. Reprod Med Biol. 2017. PMID: 29259455 Free PMC article. Review.
References
-
- Alastalo, T.P., Lönnström, M., Leppä, S., Kaarniranta, K., Pelto-Huikko, M., Sistonen, L., and Parvinen, M. (1998). Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell Res. 240, 16–27. - PubMed
-
- Biggiogera, M., Fakan, S., Leser, G., Martin, T.E., and Gordon, J. (1990). Immunoelectron microscopical visualization of ribonucleoproteins in the chromatoid body of mouse spermatids. Mol. Reprod. Dev. 26, 150–158. - PubMed
-
- Bohrmann, J., and Biber, K. (1994). Cytoskeleton-dependent transport of cytoplasmic particles in pre-vitellogenic to mid-vitellogenic ovarian follicles of Drosophila: time-lapse analysis using video-enhanced contrast microscopy. J. Cell Sci. 107, 849–858. - PubMed
-
- Braun, R.E., Behringer, R.R., Peschon, J.J., Brinster, R.L., and Palmiter, R.D. (1989). Genetically haploid spermatids are phenotypically diploid. Nature 337, 373–376. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

