Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles
- PMID: 12857871
- PMCID: PMC165683
- DOI: 10.1091/mbc.e03-02-0108
Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles
Abstract
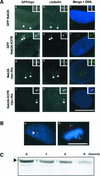
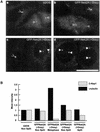
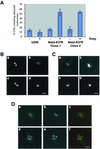
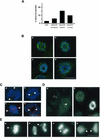
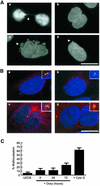
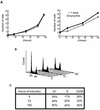
Nek2A is a cell cycle-regulated kinase of the never in mitosis A (NIMA) family that is highly enriched at the centrosome. One model for Nek2A function proposes that it regulates cohesion between the mother and daughter centriole through phosphorylation of C-Nap1, a large coiled-coil protein that localizes to centriolar ends. Phosphorylation of C-Nap1 at the G2/M transition may trigger its displacement from centrioles, promoting their separation and subsequent bipolar spindle formation. To test this model, we generated tetracycline-inducible cell lines overexpressing wild-type and kinase-dead versions of Nek2A. Live cell imaging revealed that active Nek2A stimulates the sustained splitting of interphase centrioles indicative of loss of cohesion. However, this splitting is accompanied by only a partial reduction in centriolar C-Nap1. Strikingly, induction of kinase-dead Nek2A led to formation of monopolar spindles with unseparated spindle poles that lack C-Nap1. Furthermore, kinase-dead Nek2A interfered with chromosome segregation and cytokinesis and led to an overall change in the DNA content of the cell population. These results provide the first direct evidence in human cells that Nek2A function is required for the correct execution of mitosis, most likely through promotion of centrosome disjunction. However, they suggest that loss of centriole cohesion and C-Nap1 displacement may be distinct mitotic events.
Figures



References
-
- Blangy, A., Lane, H.A., d'Herin, P., Harper, M., Kress, M., and Nigg, E.A. (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169. - PubMed
-
- Bobinnec, Y., Moudjou, M., Fouquet, J.P., Desbruyeres, E., Edde, B., and Bornens, M. (1998). Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskeleton 39, 223–232. - PubMed
-
- Bornens, M., and Moudjou, M. (1999). Studying the composition and function of centrosomes in vertebrates. Methods Cell Biol. 61, 13–34. - PubMed
-
- Bornens, M., Paintrand, M., Berges, J., Marty, M.C., and Karsenti, E. (1987). Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskeleton 8, 238–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

