Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes
- PMID: 12857877
- PMCID: PMC165689
- DOI: 10.1091/mbc.e02-11-0722
Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes
Abstract
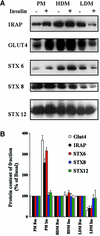
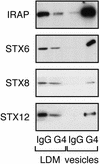
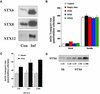
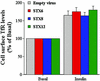
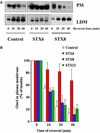
Insulin stimulates the movement of glucose transporter-4 (Glut4)-containing vesicles to the plasma membrane of adipose cells. We investigated the role of post-Golgi t-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) in the trafficking of Glut4 in 3T3-L1 adipocytes. Greater than 85% of syntaxin 6 was found in Glut4-containing vesicles, and this t-SNARE exhibited insulin-stimulated movement to the plasma membrane. In contrast, the colocalization of Glut4 with syntaxin 7, 8, or 12/13 was limited and these molecules did not translocate to the plasma membrane. We used adenovirus to overexpress the cytosolic domain of these syntaxin's and studied their effects on Glut4 traffic. Overexpression of the cytosolic domain of syntaxin 6 did not affect insulin-stimulated glucose transport, but increased basal deGlc transport and cell surface Glut4 levels. Moreover, the syntaxin 6 cytosolic domain significantly reduced the rate of Glut4 reinternalization after insulin withdrawal and perturbed subendosomal Glut4 sorting; the corresponding domains of syntaxins 8 and 12 were without effect. Our data suggest that syntaxin 6 is involved in a membrane-trafficking step that sequesters Glut4 away from traffic destined for the plasma membrane. We speculate that this is at the level of traffic of Glut4 into its unique storage compartment and that syntaxin 16 may be involved.
Figures




References
-
- Austin, C., Hinners, I., and Tooze, S.A. (2000). Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 275, 21862-21869. - PubMed
-
- Brant, A.M., Jess, T.J., Milligan, G., Brown, C.M., and Gould, G.W. 1993. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem. Biophys. Res. Commun. 192, 1297-1302. - PubMed
-
- Bryant, N.J., Govers, R., and James, D.E. (2002). Regulated trafficking of the glucose transporter, Glut4. Nat. Rev. Mol. Cell. Biol. 3, 267-277. - PubMed
-
- Byrant, N.J., Govers, R., and James, D.E. (2002). Regulated transport of the glucose transporter GLUT4. Mol. Cell. Biol. 3, 267-277. - PubMed
-
- Cain, C.C., Trimble, W.S., and Lienhard, G.E. (1992). Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J. Biol. Chem. 267, 11681-11684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

