Vaccination of mice with bacteria carrying a cloned herpesvirus genome reconstituted in vivo
- PMID: 12857893
- PMCID: PMC165264
- DOI: 10.1128/jvi.77.15.8249-8255.2003
Vaccination of mice with bacteria carrying a cloned herpesvirus genome reconstituted in vivo
Abstract
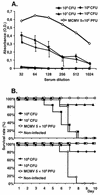
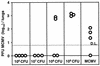
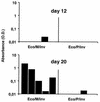
Bacterial delivery systems are gaining increasing interest as potential vaccination vectors to deliver either proteins or nucleic acids for gene expression in the recipient. Bacterial delivery systems for gene expression in vivo usually contain small multicopy plasmids. We have shown before that bacteria containing a herpesvirus bacterial artificial chromosome (BAC) can reconstitute the virus replication cycle after cocultivation with fibroblasts in vitro. In this study we addressed the question of whether bacteria containing a single plasmid with a complete viral genome can also reconstitute the viral replication process in vivo. We used a natural mouse pathogen, the murine cytomegalovirus (MCMV), whose genome has previously been cloned as a BAC in Escherichia coli. In this study, we tested a new application for BAC-cloned herpesvirus genomes. We show that the MCMV BAC can be stably maintained in certain strains of Salmonella enterica serovar Typhimurium as well and that both serovar Typhimurium and E. coli harboring the single-copy MCMV BAC can reconstitute a virus infection upon injection into mice. By this procedure, a productive virus infection is regenerated only in immunocompromised mice. Virus reconstitution in vivo causes elevated titers of specific anti-MCMV antibodies, protection against lethal MCMV challenge, and strong expression of additional genes introduced into the viral genome. Thus, the reconstitution of infectious virus from live attenuated bacteria presents a novel concept for multivalent virus vaccines launched from bacterial vectors.
Figures



Similar articles
-
Repair of an Attenuated Low-Passage Murine Cytomegalovirus Bacterial Artificial Chromosome Identifies a Novel Spliced Gene Essential for Salivary Gland Tropism.J Virol. 2020 Oct 27;94(22):e01456-20. doi: 10.1128/JVI.01456-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32847854 Free PMC article.
-
Oral Vaccination with Attenuated Salmonella Expressing Viral M25 Protein Effectively Protects Mice Against Murine Cytomegalovirus Infection.Pathogens. 2025 Mar 25;14(4):314. doi: 10.3390/pathogens14040314. Pathogens. 2025. PMID: 40333046 Free PMC article.
-
Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65).J Virol. 2000 Apr;74(8):3696-708. doi: 10.1128/jvi.74.8.3696-3708.2000. J Virol. 2000. PMID: 10729145 Free PMC article.
-
[BAC system: A novel method for manipulation of herpesvirus genomes based on bacterial genetics].Uirusu. 2004 Dec;54(2):255-64. doi: 10.2222/jsv.54.255. Uirusu. 2004. PMID: 15745165 Review. Japanese.
-
Cloning of herpesviral genomes as bacterial artificial chromosomes.Rev Med Virol. 2003 Mar-Apr;13(2):111-21. doi: 10.1002/rmv.380. Rev Med Virol. 2003. PMID: 12627394 Review.
Cited by
-
Improving Salmonella vector with rec mutation to stabilize the DNA cargoes.BMC Microbiol. 2011 Feb 8;11:31. doi: 10.1186/1471-2180-11-31. BMC Microbiol. 2011. PMID: 21303535 Free PMC article.
-
A single-dose MCMV-based vaccine elicits long-lasting immune protection in mice against distinct SARS-CoV-2 variants.Front Immunol. 2024 Jul 25;15:1383086. doi: 10.3389/fimmu.2024.1383086. eCollection 2024. Front Immunol. 2024. PMID: 39119342 Free PMC article.
-
Cytomegalovirus vaccine development.Curr Top Microbiol Immunol. 2008;325:361-82. doi: 10.1007/978-3-540-77349-8_20. Curr Top Microbiol Immunol. 2008. PMID: 18637516 Free PMC article. Review.
-
Generation of influenza virus from avian cells infected by Salmonella carrying the viral genome.PLoS One. 2015 Mar 5;10(3):e0119041. doi: 10.1371/journal.pone.0119041. eCollection 2015. PLoS One. 2015. PMID: 25742162 Free PMC article.
-
Herpesvirus BACs: past, present, and future.J Biomed Biotechnol. 2011;2011:124595. doi: 10.1155/2011/124595. Epub 2010 Oct 27. J Biomed Biotechnol. 2011. PMID: 21048927 Free PMC article. Review.
References
-
- Brune, W., M. Hasan, M. Krych, I. Bubic, S. Jonjic, and U. H. Koszinowski. 2001. Secreted virus-encoded proteins reflect murine cytomegalovirus productivity in organs. J. Infect. Dis. 184:1320-1324. - PubMed
-
- Brune, W., H. Hengel, and U. H. Koszinowski. 1999. A mouse model for cytomegalovirus infection, p. 19.7.1-19.7.13. In J. Coligan, A. Kruisbeen, D. Marguiles, E. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley, New York, N.Y. - PubMed
-
- Brune, W., C. Menard, J. Heesemann, and U. H. Koszinowski. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

