Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity
- PMID: 12857895
- PMCID: PMC165227
- DOI: 10.1128/jvi.77.15.8263-8271.2003
Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity
Abstract
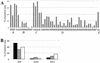
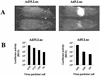
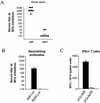
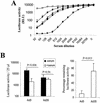
Replication-deficient human adenovirus type 5 (Ad5) can be produced to high titers in complementing cell lines, such as PER.C6, and is widely used as a vaccine and gene therapy vector. However, preexisting immunity against Ad5 hampers consistency of gene transfer, immunological responses, and vector-mediated toxicities. We report the identification of human Ad35 as a virus with low global prevalence and the generation of an Ad35 vector plasmid system for easy insertion of heterologous genes. In addition, we have identified the minimal sequence of the Ad35-E1B region (molecular weight, 55,000 [55K]), pivotal for complementation of fully E1-lacking Ad35 vector on PER.C6 cells. After stable insertion of the 55K sequence into PER.C6 cells a cell line was obtained (PER.C6/55K) that efficiently transcomplements both Ad5 and Ad35 vectors. We further demonstrate that transduction with Ad35 is not hampered by preexisting Ad5 immunity and that Ad35 efficiently infects dendritic cells, smooth muscle cells, and synoviocytes, in contrast to Ad5.
Figures

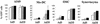
References
-
- Basler, C. F., G. Droguett, and M. S. Horwitz. 1996. Sequence of the immunoregulatory early region 3 and flanking sequences of adenovirus type 35. Gene 170:249-254. - PubMed
-
- Basler, C. F., and M. S. Horwitz. 1996. Subgroup B adenovirus type 35 early region 3 mRNAs differ from those of the subgroup C adenoviruses. Virology 215:165-177. - PubMed
-
- Boshart, M., F. Weber, G. Jahn, K. Dorsch-Häler, B. Fleckenstein, and W. Scaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41:521-530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

