Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16
- PMID: 12857908
- PMCID: PMC165259
- DOI: 10.1128/jvi.77.15.8386-8393.2003
Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16
Abstract
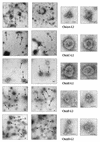
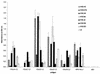
Both the Human papillomavirus (HPV) major (L1) and minor (L2) capsid proteins have been well investigated as potential vaccine candidates. The L1 protein first oligomerizes into pentamers, and these capsomers assemble into virus-like particles (VLPs) that are highly immunogenic. Here we examine the potential of using HPV type 16 (HPV-16) L1 subunits to display a well-characterized HPV-16 L2 epitope (LVEETSFIDAGAP), which is a common-neutralizing epitope for HPV types 6 and 16, in various regions of the L1 structure. The L2 sequence was introduced by PCR (by replacing 13 codons) into sequences coding for L1 surface loops D-E (chideltaC-L2), E-F (chideltaA-L2), and an internal loop C-D (chideltaH-L2); into the h4 helix (chideltaF-L2); and between h4 and beta-J structural regions (chideltaE-L2). The chimeric protein product was characterized using a panel of monoclonal antibodies (MAbs) that bind to conformational and linear epitopes, as well as a polyclonal antiserum raised to the L2 epitope. All five chimeras reacted with the L2 serum. ChideltaA-L2, chideltaE-L2, and chideltaF-L2 reacted with all the L1 antibodies, chideltaC-L2 did not bind H16:V5 and H16:E70, and chideltaH-L2 did not bind any conformation-dependent MAb. The chimeric particles elicited high-titer anti-L1 immune responses in BALB/c mice. Of the five chimeras tested only chideltaH-L2 did not elicit an L2 response, while chideltaF-L2 elicited the highest L2 response. This study provides support for the use of PV particles as vectors to deliver various epitopes in a number of locations internal to the L1 protein and for the potential of using chimeric PV particles as multivalent vaccines. Moreover, it contributes to knowledge of the structure of HPV-16 L1 VLPs and their derivatives.
Figures


Similar articles
-
Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles.J Virol. 2016 Jun 24;90(14):6314-25. doi: 10.1128/JVI.00449-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147749 Free PMC article.
-
Type-specific and cross-reactive antibodies induced by human papillomavirus 31 L1/L2 virus-like particles.J Med Microbiol. 2007 Jul;56(Pt 7):907-913. doi: 10.1099/jmm.0.47073-0. J Med Microbiol. 2007. PMID: 17577054
-
Mutations on the FG surface loop of human papillomavirus type 16 major capsid protein affect recognition by both type-specific neutralizing antibodies and cross-reactive antibodies.J Med Virol. 2005 Dec;77(4):558-65. doi: 10.1002/jmv.20492. J Med Virol. 2005. PMID: 16254978
-
Papillomavirus-like particle vaccines.J Natl Cancer Inst Monogr. 2001;(28):50-4. doi: 10.1093/oxfordjournals.jncimonographs.a024258. J Natl Cancer Inst Monogr. 2001. PMID: 11158207 Review.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
Cited by
-
Broad Cross-Protection Is Induced in Preclinical Models by a Human Papillomavirus Vaccine Composed of L1/L2 Chimeric Virus-Like Particles.J Virol. 2016 Jun 24;90(14):6314-25. doi: 10.1128/JVI.00449-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147749 Free PMC article.
-
Second-generation prophylactic HPV vaccines: successes and challenges.Expert Rev Vaccines. 2014 Feb;13(2):247-55. doi: 10.1586/14760584.2014.865523. Epub 2013 Dec 18. Expert Rev Vaccines. 2014. PMID: 24350614 Free PMC article. Review.
-
Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in Nicotiana benthamiana plants.BMC Biotechnol. 2011 Nov 15;11:106. doi: 10.1186/1472-6750-11-106. BMC Biotechnol. 2011. PMID: 22085463 Free PMC article.
-
Broad Neutralization Responses Against Oncogenic Human Papillomaviruses Induced by a Minor Capsid L2 Polytope Genetically Incorporated Into Bacterial Ferritin Nanoparticles.Front Immunol. 2020 Dec 4;11:606569. doi: 10.3389/fimmu.2020.606569. eCollection 2020. Front Immunol. 2020. PMID: 33343580 Free PMC article.
-
Broadly neutralizing antiviral responses induced by a single-molecule HPV vaccine based on thermostable thioredoxin-L2 multiepitope nanoparticles.Sci Rep. 2017 Dec 21;7(1):18000. doi: 10.1038/s41598-017-18177-1. Sci Rep. 2017. PMID: 29269879 Free PMC article.
References
-
- Breitburd, F., J. Salmon, and G. Orth. 1997. Animal models for evaluation of human papillomavirus vaccines, p. 432-445. In E. L. Franco and J. Monsonego (ed.), New developments in cervical cancer screening and prevention. Blackwell Science Ltd., Oxford, United Kingdom
-
- Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. - PubMed
-
- Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. - PubMed
-
- Chen, X. S., G. Casini, S. C. Harrison, and R. L. Garcea. 2001. Papillomavirus capsid protein expression in Escherichia coli: purification and assembly of HPV11 and HPV16 L1. J. Mol. Biol. 307:173-182. - PubMed
-
- Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

