Hantavirus-specific CD8(+)-T-cell responses in newborn mice persistently infected with Hantaan virus
- PMID: 12857910
- PMCID: PMC165253
- DOI: 10.1128/jvi.77.15.8408-8417.2003
Hantavirus-specific CD8(+)-T-cell responses in newborn mice persistently infected with Hantaan virus
Abstract
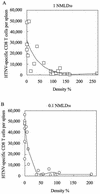
The relationship between virus-specific CD8(+)-T-cell responses and viral persistence was studied in mice by using Hantaan virus (HTNV). We first established a simple method for measuring levels of virus-specific CD8(+) T cells by flow cytometry. Next, to produce a mouse model of persistent HTNV infection, newborn mice were inoculated subcutaneously within 24 h of birth with 1 or 0.1 50% newborn mouse lethal dose of HTNV. All mice that escaped lethal infection were persistently infected with HTNV until at least 30 days after virus inoculation and had no virus-specific CD8(+) T cells producing gamma interferon (IFN-gamma). Subsequently, the virus was eliminated from some of the mice, depending on the appearance of functional virus-specific CD8(+) T cells, which have the ability to produce IFN-gamma and tumor necrosis factor alpha (TNF-alpha) and have cytotoxic activity. Neutralizing antibodies were detected in all mice, regardless of the presence or absence of virus. In the acute phase, which occurs within 30 days of infection, IFN-gamma-producing HTNV-specific CD8(+) T cells were detected on day 15 after virus inoculation. However, TNF-alpha production and the cytotoxic activity of these specific CD8(+) T cells were impaired and HTNV was not removed. Almost all of these specific CD8(+) T cells disappeared by day 18. These results suggest that functional HTNV-specific CD8(+) T cells are important for clearance of HTNV.
Figures


Similar articles
-
Age-dependent hantavirus-specific CD8(+) T-cell responses in mice infected with Hantaan virus.Arch Virol. 2004 Jul;149(7):1373-82. doi: 10.1007/s00705-003-0285-4. Epub 2004 Mar 5. Arch Virol. 2004. PMID: 15221537
-
A new model of Hantaan virus persistence in mice: the balance between HTNV infection and CD8(+) T-cell responses.Virology. 2004 May 1;322(2):318-27. doi: 10.1016/j.virol.2004.01.030. Virology. 2004. PMID: 15110529
-
CD8low CD100- T Cells Identify a Novel CD8 T Cell Subset Associated with Viral Control during Human Hantaan Virus Infection.J Virol. 2015 Dec;89(23):11834-44. doi: 10.1128/JVI.01610-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378166 Free PMC article.
-
Design and synthesis of HLA-A*02-restricted Hantaan virus multiple-antigenic peptide for CD8+ T cells.Virol J. 2020 Jan 31;17(1):15. doi: 10.1186/s12985-020-1290-x. Virol J. 2020. PMID: 32005266 Free PMC article.
-
Cellular immune response to Hantaan virus nucleocapsid protein in the acute phase of hemorrhagic fever with renal syndrome: correlation with disease severity.J Infect Dis. 2009 Jan 15;199(2):188-95. doi: 10.1086/595834. J Infect Dis. 2009. PMID: 19072554
Cited by
-
Appearance of renal hemorrhage in adult mice after inoculation of patient-derived hantavirus.Virol J. 2017 Jan 26;14(1):13. doi: 10.1186/s12985-017-0686-8. Virol J. 2017. PMID: 28122569 Free PMC article.
-
The detection of sLFA-3 in plasma of patients with hemorrhagic fever with renal syndrome.Clin Exp Med. 2009 Mar;9(1):67-71. doi: 10.1007/s10238-008-0007-z. Epub 2008 Sep 27. Clin Exp Med. 2009. PMID: 18820826
-
Immunological Responses to Seoul Orthohantavirus in Experimentally and Naturally Infected Brown Rats (Rattus norvegicus).Viruses. 2021 Apr 12;13(4):665. doi: 10.3390/v13040665. Viruses. 2021. PMID: 33921493 Free PMC article.
-
Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host.Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15502-7. doi: 10.1073/pnas.0707453104. Epub 2007 Sep 18. Proc Natl Acad Sci U S A. 2007. PMID: 17878294 Free PMC article.
-
Nlrc3 Knockout Mice Showed Renal Pathological Changes After HTNV Infection.Front Immunol. 2021 Jul 16;12:692509. doi: 10.3389/fimmu.2021.692509. eCollection 2021. Front Immunol. 2021. PMID: 34335602 Free PMC article.
References
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. (Erratum, 280:1821, 1993.) - PubMed
-
- Arikawa, J., M. Ito, J. S. Yao, H. Kariwa, I. Takashima, and N. Hashimoto. 1994. Epizootiological studies of hantavirus infection among urban rats in Hokkaido, Japan: evidences for the persistent infection from the sero-epizootiological surveys and antigenic characterizations of hantavirus isolates. J. Vet. Med. Sci. 56:27-32. - PubMed
-
- Borrow, P., and M. B. A. Oldstone. 1997. Lymphocytic choriomeningitis virus, p. 593-627. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

