Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration
- PMID: 12857948
- PMCID: PMC166424
- DOI: 10.1073/pnas.1531172100
Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration
Abstract
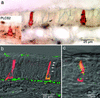
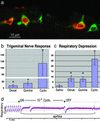
Inhalation of irritating substances leads to activation of the trigeminal nerve, triggering protective reflexes that include apnea or sneezing. Receptors for trigeminal irritants are generally assumed to be located exclusively on free nerve endings within the nasal epithelium, requiring that trigeminal irritants diffuse through the junctional barrier at the epithelial surface to activate receptors. We find, in both rats and mice, an extensive population of chemosensory cells that reach the surface of the nasal epithelium and form synaptic contacts with trigeminal afferent nerve fibers. These chemosensory cells express T2R "bitter-taste" receptors and alpha-gustducin, a G protein involved in chemosensory transduction. Functional studies indicate that bitter substances applied to the nasal epithelium activate the trigeminal nerve and evoke changes in respiratory rate. By extending to the surface of the nasal epithelium, these chemosensory cells serve to expand the repertoire of compounds that can activate trigeminal protective reflexes. The trigeminal chemoreceptor cells are likely to be remnants of the phylogenetically ancient population of solitary chemoreceptor cells found in the epithelium of all anamniote aquatic vertebrates.
Figures


References
-
- Bryant, B. P. & Silver, W. L. (2000) in The Neurobiology of Taste and Smell, eds. Finger, T. E., Silver, W. L. & Restrepo, D. (Wiley, New York), pp. 73-100.
-
- Liu, L. & Simon, S. A. (2000) Physiol. Behav. 69, 363-378. - PubMed
-
- McKemy, D. D., Neuhausser, W. M. & Julius, D. (2002) Nature 416, 52-58. - PubMed
-
- Silverman, J. D. & Kruger, L. (1989) J. Comp. Neurol. 280, 303-330. - PubMed
-
- Finger, T. E., St. Jeor, V. L., Kinnamon, J. C. & Silver, W. L. (1990) J. Comp. Neurol. 294, 293-305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

