Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates
- PMID: 12857950
- PMCID: PMC166409
- DOI: 10.1073/pnas.1530212100
Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates
Abstract
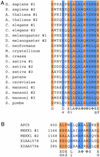
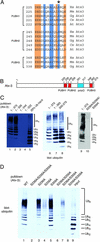
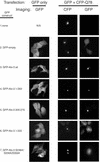
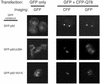
A hallmark of most neurodegenerative diseases, including those caused by polyglutamine expansion, is the formation of ubiquitin (Ub)-positive protein aggregates in affected neurons. This finding suggests that the Ub system may be involved in common mechanisms underlying these otherwise unrelated diseases. Here we report the finding of ataxin-3 (Atx-3), whose mutation is implicated in the neurodegenerative disease spinocerebellar ataxia type 3, in a bioinformatics search of the human genome for components of the Ub system. We show that wild-type Atx-3 is a Ub-binding protein and that the interaction of Atx-3 with Ub is mediated by motifs homologous to those found in a proteasome subunit. Both wild-type Atx-3 and the otherwise unrelated Ub-binding protein p62/Sequestosome-1 have been shown to be sequestered into aggregates in affected neurons in several neurodegenerative diseases, but the mechanism for this recruitment has remained unclear. In this article, we show that functional Ub-binding motifs in Atx-3 and p62 proteins are required for the localization of both proteins into aggregates in a cell-based assay that recapitulates several features of polyglutamine disease. We propose that the Ub-mediated sequestration of essential Ub-binding protein(s) into aggregates may be a common mechanism contributing to the pathogenesis of neurodegenerative diseases.
Figures

Similar articles
-
Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation.Mol Cell Biol. 2004 Sep;24(18):8055-68. doi: 10.1128/MCB.24.18.8055-8068.2004. Mol Cell Biol. 2004. PMID: 15340068 Free PMC article.
-
Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro.Hum Mol Genet. 1999 Apr;8(4):673-82. doi: 10.1093/hmg/8.4.673. Hum Mol Genet. 1999. PMID: 10072437
-
Aggregate formation inhibits proteasomal degradation of polyglutamine proteins.Hum Mol Genet. 2002 Oct 15;11(22):2689-700. doi: 10.1093/hmg/11.22.2689. Hum Mol Genet. 2002. PMID: 12374759
-
Recent advances in understanding the pathogenesis of polyglutamine diseases: involvement of molecular chaperones and ubiquitin-proteasome pathway.J Chem Neuroanat. 2003 Oct;26(2):95-101. doi: 10.1016/s0891-0618(03)00029-2. J Chem Neuroanat. 2003. PMID: 14599658 Review.
-
[Involvement of ubiquitin-proteasome system in polyglutamine disease].Tanpakushitsu Kakusan Koso. 2006 Aug;51(10 Suppl):1428-32. Tanpakushitsu Kakusan Koso. 2006. PMID: 16922413 Review. Japanese. No abstract available.
Cited by
-
Druggable genome screen identifies new regulators of the abundance and toxicity of ATXN3, the Spinocerebellar Ataxia type 3 disease protein.Neurobiol Dis. 2020 Apr;137:104697. doi: 10.1016/j.nbd.2019.104697. Epub 2019 Nov 26. Neurobiol Dis. 2020. PMID: 31783119 Free PMC article.
-
A network of protein interactions determines polyglutamine toxicity.Proc Natl Acad Sci U S A. 2006 Jul 18;103(29):11051-6. doi: 10.1073/pnas.0604548103. Epub 2006 Jul 10. Proc Natl Acad Sci U S A. 2006. PMID: 16832049 Free PMC article.
-
Activation of cannabinoid receptor type 2-induced osteogenic differentiation involves autophagy induction and p62-mediated Nrf2 deactivation.Cell Commun Signal. 2020 Jan 15;18(1):9. doi: 10.1186/s12964-020-0512-6. Cell Commun Signal. 2020. PMID: 31941496 Free PMC article.
-
Cytosolic aggregates perturb the degradation of nontranslocated secretory and membrane proteins.Mol Biol Cell. 2011 May 15;22(10):1625-37. doi: 10.1091/mbc.E10-07-0638. Epub 2011 Mar 16. Mol Biol Cell. 2011. PMID: 21411629 Free PMC article.
-
Aggresome formation and neurodegenerative diseases: therapeutic implications.Curr Med Chem. 2008;15(1):47-60. doi: 10.2174/092986708783330692. Curr Med Chem. 2008. PMID: 18220762 Free PMC article. Review.
References
-
- Glickman, M. H. & Ciechanover, A. (2002) Physiol. Rev. 82, 373–428. - PubMed
-
- Joazeiro, C. A. & Weissman, A. M. (2000) Cell 102, 549–552. - PubMed
-
- Madura, K. (2002) Cell Cycle 1, 235–244. - PubMed
-
- Donaldson, K. M., Yin, H., Gekakis, N., Supek, F. & Joazeiro, C. (2003) Curr. Biol. 13, 258–262. - PubMed
-
- Davies, B. A., Topp, J. D., Sfeir, A. J., Katzmann, D. J., Carney, D. S., Tall, G. G., Friedberg, A. S., Deng, L., Chen, Z. & Horazdovsky, B. F. (2003) J. Biol. Chem. 278, 19826–19833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

