Ca2+-independent, but voltage- and activity-dependent regulation of the NMDA receptor outward K+ current in mouse cortical neurons
- PMID: 12860921
- PMCID: PMC2343239
- DOI: 10.1113/jphysiol.2003.041723
Ca2+-independent, but voltage- and activity-dependent regulation of the NMDA receptor outward K+ current in mouse cortical neurons
Abstract
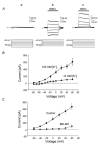
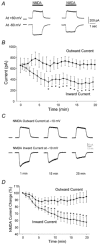
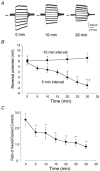
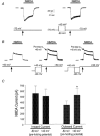
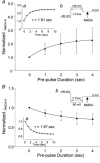
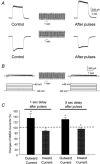
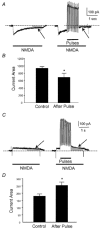
To test the novel hypothesis that the K+ efflux mediated by NMDA receptors might be regulated differently than the influx of Ca2+ and Na+ through the same receptor channels, NMDA receptor whole-cell currents carried concurrently or individually by Ca2+, Na+ and K+ were analysed in cultured mouse cortical neurons. In contrast to the NMDA inward current carried by Ca2+ and Na+, the NMDA receptor outward K+ current or NMDA-K current, recorded either in the presence or absence of extracellular Ca2+ and Na+, and at different or the same membrane potentials, showed much less sensitivity to alterations in intracellular Ca2+ concentration and underwent little rundown. In line with a selective regulation of the NMDA receptor K+ permeability, the ratio of the NMDA inward/outward currents decreased, and the reversal potential of composite NMDA currents recorded in physiological solutions shifted by -8.5 mV after repeated activation of NMDA receptors. Moreover, a depolarizing pre-pulse of a few seconds or a burst of brief depolarizing pulses selectively augmented the subsequent NMDA-K current, but not the NMDA inward current. On the other hand, a hyperpolarizing pre-pulse showed the opposite effect of reducing the NMDA-K current. The voltage- and activity-dependent regulation of the NMDA-K current did not require the existence of extracellular Ca2+ or Ca2+ influx; it was, however, affected by the duration of the pre-pulse and was subject to a time-dependent decay. The burst of excitatory activity revealed a lasting upregulation of the NMDA-K current even 5 s after termination of the pre-pulses. Our data reveal a selective regulation of the NMDA receptor K+ permeability and represent a novel model of voltage- and excitatory activity-dependent plasticity at the receptor level.
Figures

Similar articles
-
Voltage-dependent and Src-mediated regulation of NMDA receptor single channel outward currents in cortical neurons.Cell Biochem Biophys. 2007;47(2):257-70. doi: 10.1007/s12013-007-0009-6. Cell Biochem Biophys. 2007. PMID: 17652774
-
Interaction of calcium-permeable non-N-methyl-D-aspartate receptor channels with voltage-activated potassium and calcium currents in rat retinal ganglion cells in vitro.Neuroscience. 1998 Jun;84(3):877-96. doi: 10.1016/s0306-4522(97)00541-1. Neuroscience. 1998. PMID: 9579791
-
Norepinephrine selectively reduces slow Ca2+- and Na+-mediated K+ currents in cat neocortical neurons.J Neurophysiol. 1989 Feb;61(2):245-56. doi: 10.1152/jn.1989.61.2.245. J Neurophysiol. 1989. PMID: 2918353
-
The receptor-regulated calcium influx in mouse submandibular acinar cells is sodium dependent: a patch-clamp study.J Physiol. 1987 Mar;384:119-30. doi: 10.1113/jphysiol.1987.sp016446. J Physiol. 1987. PMID: 2443656 Free PMC article. Review.
-
Mechanisms of spreading depression and hypoxic spreading depression-like depolarization.Physiol Rev. 2001 Jul;81(3):1065-96. doi: 10.1152/physrev.2001.81.3.1065. Physiol Rev. 2001. PMID: 11427692 Review.
Cited by
-
Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases.Acta Pharmacol Sin. 2009 Apr;30(4):379-87. doi: 10.1038/aps.2009.24. Acta Pharmacol Sin. 2009. PMID: 19343058 Free PMC article. Review.
-
K+ efflux through postsynaptic NMDA receptors suppresses local astrocytic glutamate uptake.Glia. 2022 May;70(5):961-974. doi: 10.1002/glia.24150. Epub 2022 Jan 27. Glia. 2022. PMID: 35084774 Free PMC article.
-
Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings).CNS Neurosci Ther. 2013 Jun;19(6):370-80. doi: 10.1111/cns.12099. Epub 2013 Apr 10. CNS Neurosci Ther. 2013. PMID: 23575437 Free PMC article.
-
NMDA receptor-BK channel coupling regulates synaptic plasticity in the barrel cortex.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2107026118. doi: 10.1073/pnas.2107026118. Proc Natl Acad Sci U S A. 2021. PMID: 34453004 Free PMC article.
References
-
- Ballyk BA, Goh JW. Elevation of extracellular potassium facilitates the induction of hippocampal long-term potentiation. J Neurosci Res. 1992;33:598–604. - PubMed
-
- Bernard J, Lahsaini A, Massicotte G. Potassium-induced long-term potentiation in area CA1 of the hippocampus involves phospholipase activation. Hippocampus. 1994;4:447–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

