Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2
- PMID: 12860923
- PMCID: PMC2343328
- DOI: 10.1113/jphysiol.2003.048843
Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2
Abstract
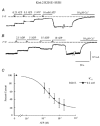
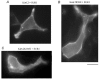
In the ATP-dependent K+ (KATP) channel pore-forming protein Kir6.2, mutation of three positively charged residues, R50, K185 and R201, impairs the ability of ATP to close the channel. The mutations do not change the channel open probability (Po) in the absence of ATP, supporting the involvement of these residues in ATP binding. We recently proposed that at least two of these positively charged residues, K185 and R201, interact with ATP phosphate groups to cause channel closure: the beta phosphate group of ATP interacts with K185 to initiate closure, while the alpha phosphate interacts with R201 to stabilize the channel's closed state. In the present study we replaced these three positive residues with residues of different charge, size and hydropathy. For K185 and R201, we found that charge, more than any other property, controls the interaction of ATP with Kir6.2. At these positions, replacement with another positive residue had minor effects on ATP sensitivity. In contrast, replacement of K185 with a negative residue (K185D/E) decreased ATP sensitivity much more than neutral substitutions, suggesting that an electrostatic interaction between the beta phosphate group of ATP and K185 destabilizes the open state of the channel. At R201, replacement with a negative charge (R201E) had multiple effects, decreasing ATP sensitivity and preventing full channel closure at high concentrations. In contrast, the R50E mutation had a modest effect on ATP sensitivity, and only residues such as proline and glycine that affect protein structure caused major decreases in ATP sensitivity at the R50 position. Based on these results and the recently published structure of Kir3.1 cytoplasmic domain, we propose a scheme where binding of the beta phosphate group of ATP to K185 induces a motion of the surrounding region, which destabilizes the open state, favouring closure of the M2 gate. Binding of the alpha phosphate group of ATP to R201 then stabilizes the closed state. R50 on the N-terminus controls ATP binding by facilitating the interaction of the beta phosphate group of ATP with K185 to destabilize the open state.
Figures



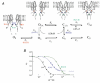

References
-
- Appel RD, Bairoch A, Hochstrasser DF. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem Sci. 1994;19:258–260. - PubMed
-
- Graham FL, van der Eb AJ. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;52:456–467. - PubMed
-
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

