CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2
- PMID: 12861003
- PMCID: PMC165719
- DOI: 10.1128/MCB.23.15.5165-5173.2003
CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2
Abstract
CDK9 is a CDC2-related kinase and the catalytic subunit of the positive-transcription elongation factor b and the Tat-activating kinase. It has recently been reported that CDK9 is a short-lived protein whose levels are regulated during the cell cycle by the SCF(SKP2) ubiquitin ligase complex (R. E. Kiernan et al., Mol. Cell. Biol. 21:7956-7970, 2001). The results presented here are in contrast to those observations. CDK9 protein levels remained unchanged in human cells entering and progressing through the cell cycle from G(0), despite dramatic changes in SKP2 expression. CDK9 levels also remained unchanged in cells exiting from mitosis and progressing through the next cell cycle. Similarly, the levels of CDK9 protein did not change as cells exited the cell cycle and differentiated along various lineages. In keeping with these observations, the kinase activity associated with CDK9 was found to not be regulated during the cell cycle. We have also found that endogenous CDK9 is a very stable protein with a half-life (t(1/2)) of 4 to 7 h, depending on the cell type. In contrast, when CDK9 is overexpressed, it is not stabilized and is rapidly degraded, with a t(1/2) of less than 1 h, depending on the level of expression. Treatment of cells with proteasome inhibitors blocked the degradation of short-lived proteins, such as p27, but did not affect the expression of endogenous CDK9. Ectopic overexpression of SKP2 led to reduction of p27 protein levels but had no effect on the expression of endogenous CDK9. Finally, downregulation of endogenous SKP2 gene expression by interfering RNA had no effect on CDK9 protein levels, whereas p27 protein levels increased dramatically. Therefore, the SCF(SKP2) ubiquitin ligase does not regulate CDK9 expression in a cell cycle-dependent manner.
Figures
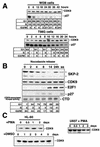
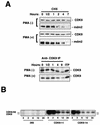

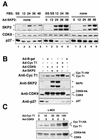


References
-
- Ausubel, F. M., R. Brent, R. E. Kington, D. D. Moore, J. G. Seidman, J. A. Smith, and K. E. Struhl. 1988. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
-
- Barboric, M., R. M. Nissen, S. Kanazawa, N. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-κB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8:327-337. - PubMed
-
- Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43:161-189. - PubMed
-
- Bhattacharya, S., J. Garriga, J. Calbó, T. Yong, D. S. Haines, and X. Graña. 2003. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene 22:2443-2451. - PubMed
-
- Brummelkamp, T., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
