Direct repression of splicing by transformer-2
- PMID: 12861004
- PMCID: PMC165737
- DOI: 10.1128/MCB.23.15.5174-5185.2003
Direct repression of splicing by transformer-2
Abstract
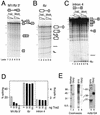
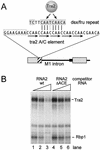
The Drosophila melanogaster sex determination factor Tra2 positively regulates the splicing of both doublesex (dsx) and fruitless (fru) pre-mRNAs but negatively affects the splicing of the M1 intron in tra2 pre-mRNA. Retention of the M1 intron is known to be part of a negative-feedback mechanism wherein the Tra2 protein limits its own synthesis, but the mechanism responsible for accumulation of M1-containing RNA is unknown. Here we show that the recombinant Tra2 protein specifically represses M1 splicing in Drosophila nuclear extracts. We find that the Tra2 protein binds directly to several sites in and near the M1 intron and that, when Tra2 binding is competed with other RNAs, the splicing of M1 is restored. Mapping the RNA sequences functionally required for M1 repression identified both a 34-nucleotide (nt) A/C-rich sequence immediately upstream of the M1 5' splice site and a region within the intron itself. The AC-rich sequence is largely composed of a repeated 4-nt sequence that also forms a subrepeat within the repeated 13-nt splicing enhancer elements of fru and dsx RNAs. Although required for repression, the element also enhances M1 splicing in the absence of Tra2. We propose that Tra2 represses M1 splicing by interacting with multiple sequences in the pre-mRNA and interfering with enhancer function.
Figures


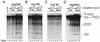

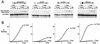
Similar articles
-
Half pint/Puf68 is required for negative regulation of splicing by the SR splicing factor Transformer2.RNA Biol. 2013 Aug;10(8):1396-406. doi: 10.4161/rna.25645. Epub 2013 Jul 9. RNA Biol. 2013. PMID: 23880637 Free PMC article.
-
The doublesex splicing enhancer components Tra2 and Rbp1 also repress splicing through an intronic silencer.Mol Cell Biol. 2007 Jan;27(2):699-708. doi: 10.1128/MCB.01572-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101798 Free PMC article.
-
Concentration dependent selection of targets by an SR splicing regulator results in tissue-specific RNA processing.Nucleic Acids Res. 2006;34(21):6256-63. doi: 10.1093/nar/gkl755. Epub 2006 Nov 10. Nucleic Acids Res. 2006. PMID: 17098939 Free PMC article.
-
Tra2 protein biology and mechanisms of splicing control.Biochem Soc Trans. 2014 Aug;42(4):1152-8. doi: 10.1042/BST20140075. Biochem Soc Trans. 2014. PMID: 25110018 Review.
-
TRA2: The dominant power of alternative splicing in tumors.Heliyon. 2023 Apr 15;9(4):e15516. doi: 10.1016/j.heliyon.2023.e15516. eCollection 2023 Apr. Heliyon. 2023. PMID: 37151663 Free PMC article. Review.
Cited by
-
Half pint/Puf68 is required for negative regulation of splicing by the SR splicing factor Transformer2.RNA Biol. 2013 Aug;10(8):1396-406. doi: 10.4161/rna.25645. Epub 2013 Jul 9. RNA Biol. 2013. PMID: 23880637 Free PMC article.
-
Identification of a splicing enhancer in MLH1 using COMPARE, a new assay for determination of relative RNA splicing efficiencies.Hum Mol Genet. 2006 Jan 15;15(2):329-36. doi: 10.1093/hmg/ddi450. Epub 2005 Dec 15. Hum Mol Genet. 2006. PMID: 16357104 Free PMC article.
-
Activation and repression functions of an SR splicing regulator depend on exonic versus intronic-binding position.Nucleic Acids Res. 2012 Jan;40(1):428-37. doi: 10.1093/nar/gkr713. Epub 2011 Sep 13. Nucleic Acids Res. 2012. PMID: 21914724 Free PMC article.
-
The doublesex splicing enhancer components Tra2 and Rbp1 also repress splicing through an intronic silencer.Mol Cell Biol. 2007 Jan;27(2):699-708. doi: 10.1128/MCB.01572-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101798 Free PMC article.
-
The Nix locus on the male-specific homologue of chromosome 1 in Aedes albopictus is a strong candidate for a male-determining factor.Parasit Vectors. 2018 Dec 24;11(Suppl 2):647. doi: 10.1186/s13071-018-3215-8. Parasit Vectors. 2018. PMID: 30583734 Free PMC article.
References
-
- Baker, B. S. 1989. Sex in flies: the splice of life. Nature 340:521-524. - PubMed
-
- Bell, L. R., J. I. Horabin, P. Schedl, and T. W. Cline. 1991. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65:229-239. - PubMed
-
- Beyer, A. L., and Y. N. Osheim. 1988. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 2:754-765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
