The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes
- PMID: 12861011
- PMCID: PMC165717
- DOI: 10.1128/MCB.23.15.5245-5255.2003
The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes
Abstract
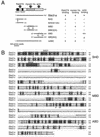
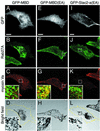
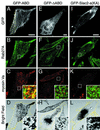
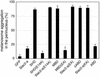
Melanosomes containing melanin pigments are transported from the cell body of melanocytes to the tips of their dendrites by a combination of microtubule- and actin-dependent machinery. Three proteins, Rab27A, myosin Va, and Slac2-a/melanophilin (a linker protein between Rab27A and myosin Va), are known to be essential for proper actin-based melanosome transport in melanocytes. Although Slac2-a directly interacts with Rab27A and myosin Va via its N-terminal region (amino acids 1 to 146) and the middle region (amino acids 241 to 405), respectively, the functional importance of the putative actin-binding domain of the Slac2-a C terminus (amino acids 401 to 590) in melanosome transport has never been elucidated. In this study we showed that formation of a tripartite protein complex between Rab27A, Slac2-a, and myosin Va alone is insufficient for peripheral distribution of melanosomes in melanocytes and that the C-terminal actin-binding domain of Slac2-a is also required for proper melanosome transport. When a Slac2-a deletion mutant (DeltaABD) or point mutant (KA) that lacks actin-binding ability was expressed in melanocytes, the Slac2-a mutants induced melanosome accumulation in the perinuclear region, possibly by a dominant negative effect, the same as the Rab27A-binding-defective mutant of Slac2-a or the myosin Va-binding-defective mutant. Our findings indicate that Slac2-a organizes actin-based melanosome transport in cooperation with Rab27A, myosin Va, and actin.
Figures




References
-
- Bahadoran, P., R. Buscà, C. Chiaverini, W. Westbroek, J. Lambert, K. Bille, G. Valony, M. Fukuda, J. M. Naeyaert, J. P. Ortonne, and R. Ballotti. 2003. Characterisation of the molecular defects in RAB27A, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J. Biol. Chem. 278:11386-11392. - PubMed
-
- Bennett, D. C., P. J. Cooper, and I. R. Hart. 1987. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer 39:414-418. - PubMed
-
- Bock, J. B., H. T. Matern, A. A. Peden, and R. H. Scheller. 2001. A genomic perspective on membrane compartment organization. Nature 409:839-841. - PubMed
-
- Chen, D., J. Guo, T. Miki, M. Tachibana, and W. A. Gahl. 1997. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 60:27-37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
