RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization
- PMID: 12861019
- PMCID: PMC165736
- DOI: 10.1128/MCB.23.15.5331-5345.2003
RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization
Abstract
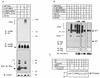
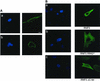
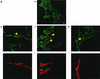
RNF5 is a RING finger protein found to be important in the growth and development of Caenorhabditis elegans. The search for RNF5-associated proteins via a yeast two-hybrid screen identified a LIM-containing protein in C. elegans which shows homology with human paxillin. Here we demonstrate that the human homologue of RNF5 associates with the amino-terminal domain of paxillin, resulting in its ubiquitination. RNF5 requires intact RING and C-terminal domains to mediate paxillin ubiquitination. Whereas RNF5 mediates efficient ubiquitination of paxillin in vivo, protein extracts were required for in vitro ubiquitination, suggesting that additional modifications and/or an associated E3 ligase assist RNF5 targeting of paxillin ubiquitination. Mutant Ubc13 efficiently inhibits RNF5 ubiquitination, suggesting that RNF5 generates polychain ubiquitin of the K63 topology. Expression of RNF5 increases the cytoplasmic distribution of paxillin while decreasing its localization within focal adhesions, where it is primarily seen under normal growth. Concomitantly, RNF5 expression results in inhibition of cell motility. Via targeting of paxillin ubiquitination, which alters its localization, RNF5 emerges as a novel regulator of cell motility.
Figures









References
-
- Borden, K. L. B. 2000. RING domains: master builders of molecular scaffolds? J. Biol. Chem. 295:1103-1112. - PubMed
-
- Brown, M. C., M. S. Curtis, and C. E. Turner. 1998. Paxillin LD motifs may define a new family of protein recognition domains. Nat. Struct. Biol. 5:677-678. - PubMed
-
- Deshaies, R. J. 1999. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell. Dev. Biol. 15:435-467. - PubMed
-
- Fuchs, S. Y., B. Xie, V. Adler, V. A. Fried, R. J. Davis, and Z. Ronai. 1997. c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J. Biol. Chem. 272:32163-32168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
