Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein
- PMID: 12861021
- PMCID: PMC165724
- DOI: 10.1128/MCB.23.15.5354-5365.2003
Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein
Abstract
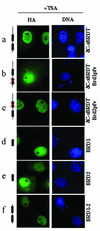
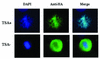
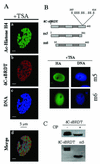
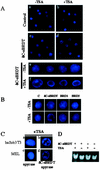
The association between histone acetylation and replacement observed during spermatogenesis prompted us to consider the testis as a source for potential factors capable of remodelling acetylated chromatin. A systematic search of data banks for open reading frames encoding testis-specific bromodomain-containing proteins focused our attention on BRDT, a testis-specific protein of unknown function containing two bromodomains. BRDT specifically binds hyperacetylated histone H4 tail depending on the integrity of both bromodomains. Moreover, in somatic cells, the ectopic expression of BRDT triggered a dramatic reorganization of the chromatin only after induction of histone hyperacetylation by trichostatin A (TSA). We then defined critical domains of BRDT involved in its activity. Both bromodomains of BRDT, as well as flanking regions, were found indispensable for its histone acetylation-dependent remodelling activity. Interestingly, we also observed that recombinant BRDT was capable of inducing reorganization of the chromatin of isolated nuclei in vitro only when the nuclei were from TSA-treated cells. This assay also allowed us to show that the action of BRDT was ATP independent, suggesting a structural role for the protein in the remodelling of acetylated chromatin. This is the first demonstration of a large-scale reorganization of acetylated chromatin induced by a specific factor.
Figures



References
-
- Agalioti, T., G. Chen, and D. Thanos. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111:381-392. - PubMed
-
- Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120-124. - PubMed
-
- Bellvé, A. R. 1993. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 225:84-113. - PubMed
-
- Christensen, M. E., and G. H. Dixon. 1982. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev. Biol. 93:404-415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
