Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells
- PMID: 12861027
- PMCID: PMC165738
- DOI: 10.1128/MCB.23.15.5421-5430.2003
Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells
Abstract
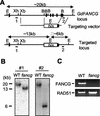
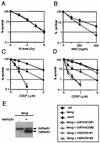
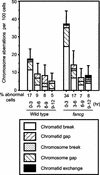
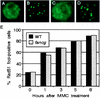
The rare hereditary disorder Fanconi anemia (FA) is characterized by progressive bone marrow failure, congenital skeletal abnormality, elevated susceptibility to cancer, and cellular hypersensitivity to DNA cross-linking chemicals and sometimes other DNA-damaging agents. Molecular cloning identified six causative genes (FANCA, -C, -D2, -E, -F, and -G) encoding a multiprotein complex whose precise biochemical function remains elusive. Recent studies implicate this complex in DNA damage responses that are linked to the breast cancer susceptibility proteins BRCA1 and BRCA2. Mutations in BRCA2, which participates in homologous recombination (HR), are the underlying cause in some FA patients. To elucidate the roles of FA genes in HR, we disrupted the FANCG/XRCC9 locus in the chicken B-cell line DT40. FANCG-deficient DT40 cells resemble mammalian fancg mutants in that they are sensitive to killing by cisplatin and mitomycin C (MMC) and exhibit increased MMC and radiation-induced chromosome breakage. We find that the repair of I-SceI-induced chromosomal double-strand breaks (DSBs) by HR is decreased approximately 9-fold in fancg cells compared with the parental and FANCG-complemented cells. In addition, the efficiency of gene targeting is mildly decreased in FANCG-deficient cells, but depends on the specific locus. We conclude that FANCG is required for efficient HR-mediated repair of at least some types of DSBs.
Figures



Similar articles
-
Direct interaction of the Fanconi anaemia protein FANCG with BRCA2/FANCD1.Hum Mol Genet. 2003 Oct 1;12(19):2503-10. doi: 10.1093/hmg/ddg266. Epub 2003 Aug 5. Hum Mol Genet. 2003. PMID: 12915460
-
Resistance to mitomycin C requires direct interaction between the Fanconi anemia proteins FANCA and FANCG in the nucleus through an arginine-rich domain.J Biol Chem. 1999 Nov 26;274(48):34212-8. doi: 10.1074/jbc.274.48.34212. J Biol Chem. 1999. PMID: 10567393
-
The Chinese hamster FANCG/XRCC9 mutant NM3 fails to express the monoubiquitinated form of the FANCD2 protein, is hypersensitive to a range of DNA damaging agents and exhibits a normal level of spontaneous sister chromatid exchange.Carcinogenesis. 2001 Dec;22(12):1939-46. doi: 10.1093/carcin/22.12.1939. Carcinogenesis. 2001. PMID: 11751423
-
Current knowledge on the pathophysiology of Fanconi anemia: from genes to phenotypes.Int J Hematol. 2001 Jul;74(1):33-41. doi: 10.1007/BF02982547. Int J Hematol. 2001. PMID: 11530803 Review.
-
Molecular pathogenesis of fanconi anemia.Int J Hematol. 2002 Feb;75(2):123-8. doi: 10.1007/BF02982016. Int J Hematol. 2002. PMID: 11939257 Review.
Cited by
-
Impaired removal of DNA interstrand cross-link in Nijmegen breakage syndrome and Fanconi anemia, but not in BRCA-defective group.Cancer Sci. 2008 Nov;99(11):2238-43. doi: 10.1111/j.1349-7006.2008.00915.x. Epub 2008 Sep 1. Cancer Sci. 2008. PMID: 18771529 Free PMC article.
-
Regulation and roles of Cdc7 kinase under replication stress.Cell Cycle. 2014;13(12):1859-66. doi: 10.4161/cc.29251. Epub 2014 May 19. Cell Cycle. 2014. PMID: 24841992 Free PMC article. Review.
-
Checkpoint kinase 1 negatively regulates somatic hypermutation.Nucleic Acids Res. 2014 Apr;42(6):3666-74. doi: 10.1093/nar/gkt1378. Epub 2014 Jan 13. Nucleic Acids Res. 2014. PMID: 24423870 Free PMC article.
-
FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway.Nat Struct Mol Biol. 2008 Nov;15(11):1138-46. doi: 10.1038/nsmb.1504. Epub 2008 Oct 19. Nat Struct Mol Biol. 2008. PMID: 18931676 Free PMC article.
-
Interaction between DNA Polymerase β and BRCA1.PLoS One. 2013 Jun 27;8(6):e66801. doi: 10.1371/journal.pone.0066801. Print 2013. PLoS One. 2013. PMID: 23826138 Free PMC article.
References
-
- Ahmad, S. I., F. Hanaoka, and S. H. Kirk. 2002. Molecular biology of Fanconi anaemia—an old problem, a new insight. Bioessays 24:439-448. - PubMed
-
- Auerbach, A., M. Buchwald, and H. Joenje. 2001. Fanconi anemia, p. 753-768. In C. Scriver, A. Beaudet, W. Sly, D. Valle, B. Childs, K. Kinzler, and B. Vogelstein (ed.), The metabolic and molecular bases of inherited diseases, 4th ed. McGraw-Hill, New York, N.Y.
-
- Bigelow, S. B., J. M. Rary, and M. A. Bender. 1979. G2 chromosomal radiosensitivity in Fanconi's anemia. Mutat. Res. 63:189-199. - PubMed
-
- Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. - PubMed
-
- Cromie, G. A., J. C. Connelly, and D. R. Leach. 2001. Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Mol. Cell 8:1163-1174. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
