Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis
- PMID: 12865407
- PMCID: PMC164290
- DOI: 10.1172/JCI17778
Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis
Abstract
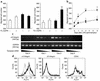
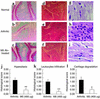
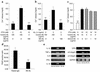
It has been shown that osteopontin (OPN) plays a pivotal role in the pathogenesis of rheumatoid arthritis (RA). However, the molecular mechanism of OPN action is yet to be elucidated. Splenic monocytes obtained from arthritic mice exhibited a significant capacity for cell migration toward thrombin-cleaved OPN but not toward full-length OPN. Migratory monocytes expressed alpha9 and alpha4 integrins. Since cleavage of OPN by thrombin exposes the cryptic epitope recognized by alpha9 and alpha4 integrins, we investigated the role of the cryptic epitope SLAYGLR in a murine RA model by using a specific antibody (M5) reacting to SLAYGLR sequence. The M5 antibody could abrogate monocyte migration toward the thrombin-cleaved form of OPN. Importantly, M5 antibody could inhibit the proliferation of synovium, bone erosion, and inflammatory cell infiltration in arthritic joints. Thus, we demonstrated that a cryptic epitope, the SLAYGLR sequence of murine OPN, is critically involved in the pathogenesis of a murine model of RA.
Figures
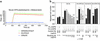

Comment in
-
Osteopontin: a bridge between bone and the immune system.J Clin Invest. 2003 Jul;112(2):147-9. doi: 10.1172/JCI19190. J Clin Invest. 2003. PMID: 12865402 Free PMC article. Review.
References
-
- van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br. J. Rheumatol. 1995;34 (Suppl. 2):74–78. - PubMed
-
- Bathon JM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N. Engl. J. Med. 2000;343:1586–1593. - PubMed
-
- Campion GV, Lebsack ME, Lookabaugh J, Gordon G, Catalano M. Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis. The IL-1Ra Arthritis Study Group. Arthritis Rheum. 1996;39:1092–1101. - PubMed
-
- Paget SA. Efficacy of anakinra in bone: comparison to other biologics. Adv. Ther. 2002;19:27–39. - PubMed
-
- Genovese MC, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: Two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–1450. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

