Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity
- PMID: 12865506
- PMCID: PMC2343277
- DOI: 10.1113/jphysiol.2003.046847
Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity
Abstract
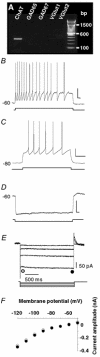
The medial septum-diagonal band complex (MSDB) contains cholinergic and non-cholinergic neurons known to play key roles in learning and memory processing, and in the generation of hippocampal theta rhythm. Electrophysiologically, several classes of neurons have been described in the MSDB, but their chemical identity remains to be fully established. By combining electrophysiology with single-cell RT-PCR, we have identified four classes of neurons in the MSDB in vitro. The first class displayed slow-firing and little or no Ih, and expressed choline acetyl-transferase mRNA (ChAT). The second class was fast-firing, had a substantial Ih and expressed glutamic acid decarboxylase 67 mRNA (GAD67), sometimes co-localized with ChAT mRNAs. A third class exhibited fast- and burst-firing, had an important Ih and expressed GAD67 mRNA also occasionally co-localized with ChAT mRNAs. The ionic mechanism underlying the bursts involved a low-threshold spike and a prominent Ih current, conductances often associated with pacemaker activity. Interestingly, we identified a fourth class that expressed transcripts solely for one or two of the vesicular glutamate transporters (VGLUT1 and VGLUT2), but not ChAT or GAD. Some putative glutamatergic neurons displayed electrophysiological properties similar to ChAT-positive slow-firing neurons such as the occurrence of a very small Ih, but nearly half of glutamatergic neurons exhibited cluster firing with intrinsically generated voltage-dependent subthreshold membrane oscillations. Neurons belonging to each of the four described classes were found among septohippocampal neurons by retrograde labelling. We provide results suggesting that slow-firing cholinergic, fast-firing and burst-firing GABAergic, and cluster-firing glutamatergic neurons, may each uniquely contribute to hippocampal rhythmicity in vivo.
Figures







References
-
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septo-hippocampal cholinergic system. J Neurophysiol. 1998;79:1633–1642. - PubMed
-
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. - PubMed
-
- Bonansco C, Buno W. Cellular mechanisms underlying the rhythmic bursts induced by NMDA microiontophoresis at the apical dendrites of CA1 pyramidal neurons. Hippocampus. 2003;13:150–163. - PubMed
-
- Brashear HR, Zaborsky L, Heimer L. Distribution of GABAergic and cholinergic neurons in the rat diagonal band. Neuroscience. 1986;17:439–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

