Photodynamic therapy effect of m-THPC (Foscan) in vivo: correlation with pharmacokinetics
- PMID: 12865935
- PMCID: PMC2394256
- DOI: 10.1038/sj.bjc.6601101
Photodynamic therapy effect of m-THPC (Foscan) in vivo: correlation with pharmacokinetics
Abstract
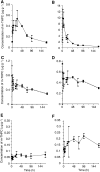
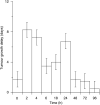
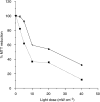
m-Tetra(hydroxyphenyl)chlorin (m-THPC, Foscan, Temoporfin) has an unusually high photodynamic efficacy which cannot be explained by its photochemical properties alone. In vivo interactions are therefore of critical importance in determining this high potency. The pharmacokinetics of m-THPC in a rat tumour model was determined using (14)C m-THPC in an LSBD(1) fibrosarcoma implanted into BDIX rats. The photodynamic therapy (PDT) efficacy was determined at different drug administrations to light intervals and correlated with the tumour and plasma pharmacokinetic data. The plasma pharmacokinetics of m-THPC can be interpreted by compartmental analysis as having three half-lives of 0.46, 6.91 and 82.5 h, with a small initial volume of distribution, suggesting retention in the vascular compartment. Tissues of the reticuloendothelial system showed high accumulation of m-THPC, particularly the liver. PDT efficacy of m-THPC over the same time course seemed to exhibit two peaks of activity (2 and 24 h), in terms of tumour growth delay with the peak at 24 h postinjection correlating to the maximum tumour concentration. Investigation on tumour cells isolated from m-THPC-treated tumours suggested that the peak PDT activity at 2 h represents an effect on the vasculature while the peak at 24 h shows a more direct response. These results indicate that the in vivo PDT effect of m-THPC occurs via several mechanisms.
Figures

Similar articles
-
Improved in vivo delivery of m-THPC via pegylated liposomes for use in photodynamic therapy.J Control Release. 2012 Jan 30;157(2):196-205. doi: 10.1016/j.jconrel.2011.09.085. Epub 2011 Oct 1. J Control Release. 2012. PMID: 21982898
-
The high photoactivity of m-THPC in photodynamic therapy. Unusually strong retention of m-THPC by RIF-1 cells in culture.Photochem Photobiol. 1999 Mar;69(3):360-3. doi: 10.1562/0031-8655(1999)069<0360:thpoti>2.3.co;2. Photochem Photobiol. 1999. PMID: 10089829
-
Pharmacokinetics of a tri-glucoconjugated 5,10,15-(meta)-trihydroxyphenyl-20-phenyl porphyrin photosensitizer for PDT. A single dose study in the rat.J Photochem Photobiol B. 2006 Oct 2;85(1):56-64. doi: 10.1016/j.jphotobiol.2006.03.008. Epub 2006 Jun 12. J Photochem Photobiol B. 2006. PMID: 16765603
-
Temoporfin (Foscan®, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)--a second-generation photosensitizer.Photochem Photobiol. 2011 Nov-Dec;87(6):1240-96. doi: 10.1111/j.1751-1097.2011.00986.x. Epub 2011 Sep 19. Photochem Photobiol. 2011. PMID: 21848905 Review.
-
The Photosensitizer Temoporfin (mTHPC) - Chemical, Pre-clinical and Clinical Developments in the Last Decade.Photochem Photobiol. 2023 Mar;99(2):356-419. doi: 10.1111/php.13730. Epub 2022 Nov 3. Photochem Photobiol. 2023. PMID: 36161310 Review.
Cited by
-
In vivo quantification of photosensitizer fluorescence in the skin-fold observation chamber using dual-wavelength excitation and NIR imaging.Lasers Med Sci. 2011 Nov;26(6):789-801. doi: 10.1007/s10103-011-0888-z. Epub 2011 Jan 29. Lasers Med Sci. 2011. PMID: 21279401 Free PMC article.
-
Compartmental targeting for mTHPC-based photodynamic treatment in vivo: Correlation of efficiency, pharmacokinetics, and regional distribution of apoptosis.Int J Radiat Oncol Biol Phys. 2010 Oct 1;78(2):563-71. doi: 10.1016/j.ijrobp.2010.04.009. Epub 2010 Jul 23. Int J Radiat Oncol Biol Phys. 2010. PMID: 20656417 Free PMC article.
-
Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers.Chem Biol Drug Des. 2017 Feb;89(2):192-206. doi: 10.1111/cbdd.12792. Chem Biol Drug Des. 2017. PMID: 28205400 Free PMC article. Review.
-
Therapeutic Options for Recurrent Glioblastoma-Efficacy of Talaporfin Sodium Mediated Photodynamic Therapy.Pharmaceutics. 2022 Feb 2;14(2):353. doi: 10.3390/pharmaceutics14020353. Pharmaceutics. 2022. PMID: 35214085 Free PMC article.
-
Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin.Br J Cancer. 2005 Jun 6;92(11):2004-9. doi: 10.1038/sj.bjc.6602600. Br J Cancer. 2005. PMID: 15886704 Free PMC article.
References
-
- Ball DJ, Vernon DI, Brown SB (1999) The high photoactivity of m-THPC in photodynamic therapy. Unusually strong retention of m-THPC by RIF-1 cells in culture. Photochem Photobiol 69: 360–363 - PubMed
-
- Blant SA, Glanzmann TM, Ballini J-P, Wagnieres G, van den Bergh H, Monier P (2002) Uptake and localisation of mTHPC (Foscan®) and its 14C-labelled form in normal and tumour tissues of the hamster squamous cell carcinoma model: a comparative study. Br J Cancer 87: 1470–1478, doi:10.1038/sj.bjc.6600651 - PMC - PubMed
-
- Boyle RW, Dolphin D (1996) Invited review: structure and biodistribution relationships of photodynamic sensitizers. Photochem Photobiol 64: 469–485 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

