CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors
- PMID: 12867498
- PMCID: PMC6740553
- DOI: 10.1523/JNEUROSCI.23-15-06163.2003
CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors
Erratum in
- J Neurosci. 2003 Sep 3;23(22):following 8158. Figueredo, HF [corrected to Figueiredo, HF]
Abstract
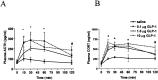
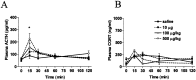
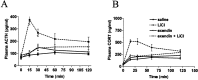
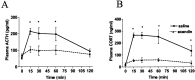
Responses to stressors serve to adjust physiology and behavior to increase short-term survival at the potential expense of increasing susceptibility to disease over the long term. We show that glucagon-like peptide-1 (7-36) amide (GLP-1) increases levels of the stress-activated hormones ACTH and corticosterone when administered directly into the rat brain and increases levels of anxiety as measured by the elevated plus maze. The endocrine response is preferentially activated by GLP-1 administration in the paraventricular nucleus of the hypothalamus, whereas the anxiety response is preferentially activated by administration in the central nucleus of the amygdala. Furthermore, GLP-1 antagonists block increases in stress hormones associated with the toxin LiCl and both the endocrine and anxiety responses to vertical heights. Although diverse neural circuits must necessarily process disparate stressors, the current data implicate a role for the GLP-1 system as a critical mediator of multiple stress responses.
Figures


References
-
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M ( 1993) Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry 50: 295–305. - PubMed
-
- Drucker DJ ( 1990) Glucagon and the glucagon-like peptides. Pancreas 5: 484–488. - PubMed
-
- Goke R, Larsen PJ, Mikkelsen JD, Skeikh SP ( 1995) Distribution of GLP-1 binding sites in the rat brain: evidence that exendin is a ligand of brain GLP-1 sites. Eur J Neurosci 7: 2294–2300. - PubMed
-
- Grillon C, Southwick SM, Charney DS ( 1996) The psychobiological basis of posttraumatic stress disorder: association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry 1: 278–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
