Tenascin-R as a repellent guidance molecule for developing optic axons in zebrafish
- PMID: 12867507
- PMCID: PMC6740549
- DOI: 10.1523/JNEUROSCI.23-15-06232.2003
Tenascin-R as a repellent guidance molecule for developing optic axons in zebrafish
Abstract
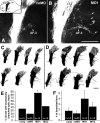
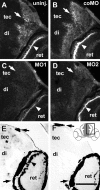
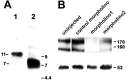
To investigate the role of tenascin-R in nervous system development, we studied axon pathfinding in the developing optic system of zebrafish. Zebrafish tenascin-R has the same domain structure as tenascin-R in amniotes. Amino acid sequence identity with human tenascin-R is 60%. In 3-d-old larvae, tenascin-R mRNA is expressed in scattered cells throughout the periventricular cell layer of the diencephalon and tectum. Tenascin-R immunoreactivity is not detectable in the optic nerve, optic tract, or tectal optic neuropil but immediately borders the optic tract caudally. Reducing expression of tenascin-R in 3-d-old larvae in vivo by injecting morpholinos into fertilized eggs led to excessive branching of the optic tract in 86% of all injected larvae compared with 20-37% in controls. Branches were almost exclusively caudal, where tenascin-R immunoreactivity normally borders the optic tract, suggesting a role for tenascin-R in guiding optic axons in the ventral diencephalon by a contact-repellent mechanism.
Figures

References
-
- Bartsch U, Pesheva P, Raff M, Schachner M ( 1993) Expression of janusin (J1–160/180) in the retina and optic nerve of the developing and adult mouse. Glia 9: 57–69. - PubMed
-
- Becker T, Anliker B, Becker CG, Taylor J, Schachner M, Meyer RL, Bartsch U ( 2000) Tenascin-R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia 29: 330–346. - PubMed
-
- Burrill JD, Easter SS ( 1994) Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio). J Comp Neurol 346: 583–600. - PubMed
-
- Dickson BJ ( 2002) Molecular mechanisms of axon guidance. Science 298: 1959–1964. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
