Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment
- PMID: 12867511
- PMCID: PMC6740545
- DOI: 10.1523/JNEUROSCI.23-15-06264.2003
Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment
Abstract
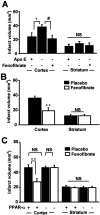
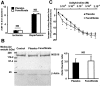
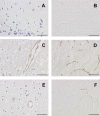
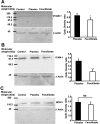
The treatment of ischemic strokes is limited to the prevention of cerebrovascular risk factors and to the modulation of the coagulation cascade during the acute phase. A new therapeutic strategy could be to preventively protect the brain against noxious biological reactions induced by cerebral ischemia such as oxidative stress and inflammation to minimize their neurological consequences. Here, we show that a peroxisome proliferator-activated receptor (PPAR-alpha) activator, fenofibrate, protects against cerebral injury by anti-oxidant and anti-inflammatory mechanisms. A 14 d preventive treatment with fenofibrate reduces susceptibility to stroke in apolipoprotein E-deficient mice as well as decreases cerebral infarct volume in C57BL/6 wild-type mice. The neuroprotective effect of fenofibrate is completely absent in PPAR-alpha-deficient mice, suggesting that PPAR-alpha activation is involved as a mechanism of the protection against cerebral injury. Furthermore, this neuroprotective effect appears independently of any improvement in plasma lipids or glycemia and is associated with (1) an improvement in middle cerebral artery sensitivity to endothelium-dependent relaxation unrelated to an increase in nitric oxide synthase (NOS) type III expression, (2) a decrease in cerebral oxidative stress depending on the increase in numerous antioxidant enzyme activities, and (3) the prevention of ischemia-induced expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in cerebral vessels without any change in NOS II expression. These data demonstrate that PPAR-alpha could be a new pharmacological target to preventively reduce the deleterious neurological consequences of stroke in mice and suggest that PPAR-alpha activators could preventively decrease the severity of stroke in humans.
Figures

Similar articles
-
The impact of single and combined PPAR-α and PPAR-γ activation on the neurological outcomes following cerebral ischemia reperfusion.Life Sci. 2020 Jul 1;252:117679. doi: 10.1016/j.lfs.2020.117679. Epub 2020 Apr 20. Life Sci. 2020. PMID: 32325134
-
Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone.J Neurochem. 2006 Apr;97(2):435-48. doi: 10.1111/j.1471-4159.2006.03758.x. Epub 2006 Mar 15. J Neurochem. 2006. PMID: 16539667
-
Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents.J Neurochem. 2007 Apr;101(1):41-56. doi: 10.1111/j.1471-4159.2006.04376.x. J Neurochem. 2007. PMID: 17394460
-
PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases.Biochem Soc Trans. 2006 Dec;34(Pt 6):1341-6. doi: 10.1042/BST0341341. Biochem Soc Trans. 2006. PMID: 17073815 Review.
-
[Cell protection through PPAR nuclear receptor activation].Therapie. 2004 Jan-Feb;59(1):25-9. doi: 10.2515/therapie:2004006. Therapie. 2004. PMID: 15199664 Review. French.
Cited by
-
Gemfibrozil pretreatment resulted in a sexually dimorphic outcome in the rat models of global cerebral ischemia-reperfusion via modulation of mitochondrial pro-survival and apoptotic cell death factors as well as MAPKs.J Mol Neurosci. 2013 Jul;50(3):379-93. doi: 10.1007/s12031-012-9932-0. Epub 2013 Jan 5. J Mol Neurosci. 2013. PMID: 23288702
-
Endothelial cells and astrocytes: a concerto en duo in ischemic pathophysiology.Int J Cell Biol. 2012;2012:176287. doi: 10.1155/2012/176287. Epub 2012 Jun 24. Int J Cell Biol. 2012. PMID: 22778741 Free PMC article.
-
Pretreatment with peroxysome proliferator-activated receptor alpha agonist fenofibrate protects endothelium in rabbit Escherichia coli endotoxin-induced shock.Intensive Care Med. 2005 Sep;31(9):1269-79. doi: 10.1007/s00134-005-2730-1. Epub 2005 Aug 16. Intensive Care Med. 2005. PMID: 16132896
-
Fasting Upregulates PPARalpha Target Genes in Brain and Influences Pituitary Hormone Expression in a PPARalpha Dependent Manner.PPAR Res. 2009;2009:801609. doi: 10.1155/2009/801609. PPAR Res. 2009. PMID: 20011657 Free PMC article.
-
Therapeutic Potential of PPARγ Activation in Stroke.PPAR Res. 2008;2008:461981. doi: 10.1155/2008/461981. Epub 2008 Apr 13. PPAR Res. 2008. PMID: 21909480 Free PMC article.
References
-
- Amarenco P ( 2001) Hypercholesterolemia, lipid-lowering agents, and the risk for brain infarction. Neurology 57[Suppl 2]: S35–44. - PubMed
-
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA ( 2001) Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke 32: 980–986. - PubMed
-
- Bastide M, Bordet R, Pu Q, Robin E, Puisieux F, Dupuis B ( 1999) Relationship between inward rectifier potassium current impairment and brain injury after cerebral ischemia/reperfusion. J Cereb Blood Flow Metab 19: 1309–1315. - PubMed
-
- Bastide M, Gelé P,Pétrault O, Pu Q, Caliez A, Robin E, Deplanque D, Duriez P, Bordet R. ( 2003) Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab 23: 399–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
