Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells
- PMID: 12867523
- PMCID: PMC6740543
- DOI: 10.1523/JNEUROSCI.23-15-06373.2003
Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells
Abstract
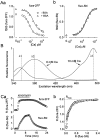
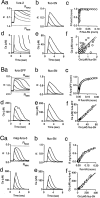
Many types of neurons release endocannabinoids from their dendrites in response to elevation of intracellular calcium levels. Endocannabinoids then activate presynaptic cannabinoid receptors, thereby inhibiting neurotransmitter release for tens of seconds. A crucial step in understanding the physiological role of this retrograde signaling is to determine its sensitivity to elevations of postsynaptic calcium. Here we determine and compare the calcium dependence of endocannabinoid-mediated retrograde inhibition at three types of synapses onto cerebellar Purkinje cells. Previous studies have shown that Purkinje cell depolarization results in endocannabinoid-mediated retrograde inhibition of synapses received from climbing fibers, granule cell parallel fibers, and inhibitory interneurons. Using several calcium indicators with a range of affinities, we performed a series of in situ and in vitro calibrations to quantify calcium levels in Purkinje cells. We found that postsynaptic calcium levels of approximately 15 microM are required for half-maximal retrograde inhibition at all of these synapses. In contrast, previous studies had suggested that endocannabinoid release could occur with slight elevations of calcium above resting levels, which implies that inhibition should be widespread and continuously modulated by subtle changes in intracellular calcium levels. However, our results indicate that such small changes in intracellular calcium are not sufficient to evoke endocannabinoid release. Instead, because of its high requirement for calcium, retrograde inhibition mediated by calcium-dependent endocannabinoid release from Purkinje cells will occur under more restricted conditions and with greater spatial localization than previously appreciated.
Figures






References
-
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D ( 1997) Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277: 1094–1097. - PubMed
-
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB ( 1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–87. - PubMed
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R ( 1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
