Phospholipid hydroperoxide glutathione peroxidase induces a delay in G1 of the cell cycle
- PMID: 12868489
- PMCID: PMC4638222
- DOI: 10.1080/1071576031000088283
Phospholipid hydroperoxide glutathione peroxidase induces a delay in G1 of the cell cycle
Abstract
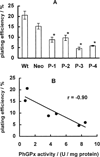
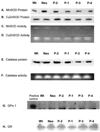
Phospholipid hydroperoxide glutathione peroxidase (PhGPx) is an antioxidant enzyme that reduces cellular phospholipid hydroperoxides (PLOOHs) to alcohols. Cellular peroxide tone has been implicated in cell growth and differentiation. By reducing the PLOOH level in the cell membrane, PhGPx regulates the peroxide tone and thereby might be involved in cell growth. We hypothesized that overexpression of PhGPx in human breast cancer cells would decrease their growth rate. We stably transfected MCF-7 cells (Wt) with L-PhGPx and measured cell doubling time, plating efficiency, and cell cycle phase transit times. P-4 cells (8-fold increase in PhGPx activity) showed a 2-fold increase in doubling time; doubling time increased directly with PhGPx activity (r = 0.95). The higher the PhGPx activity, the lower the plating efficiency (r = -0.86). The profile of other antioxidant enzymes was unchanged. Overexpression of PhGPx lowered the steady-state level of PLOOH (by > 60%). Results from bromodeoxyuridine pulse-chase experiments and flow cytometry indicate that PhGPx induced a delay in MCF-7 proliferation that was primarily due to a slower progression from G1 to S. These results support the hypothesis that PhGPx plays a regulatory role in the progression of MCF-7 cells from G1 to S possibly by regulating the steady-state levels of PLOOH. These data suggest that PhGPx can lower the peroxide tone, which might change the cellular redox environment resulting in a delay in G1 transit. Thus, PhGPx could be an important factor in cell growth.
Figures




References
-
- Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta. 1985;839:62–70. - PubMed
-
- Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation in situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 1990;265:454–461. - PubMed
-
- Pushpa-Rekha TR, Burdsall AL, Oleksa LM, Chisolm GM, Driscoll DM. Rat phospholipid-hydroperoxide glutathione peroxidase. cDNA cloning and identification of multiple transcription and translation start sites. J. Biol. Chem. 1995;270:26993–26999. - PubMed
-
- Weitzel F, Wendel A. Selenoenzymes regulate the activity of leukocyte 5-lipoxygenase via the peroxide tone. J. Biol. Chem. 1993;268:6288–6292. - PubMed
-
- Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
