Pre-neutralization of C5a-mediated effects by the monoclonal antibody 137-26 reacting with the C5a moiety of native C5 without preventing C5 cleavage
- PMID: 12869020
- PMCID: PMC1808762
- DOI: 10.1046/j.1365-2249.2003.02213.x
Pre-neutralization of C5a-mediated effects by the monoclonal antibody 137-26 reacting with the C5a moiety of native C5 without preventing C5 cleavage
Abstract
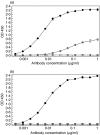
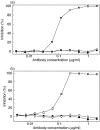
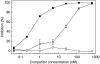
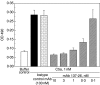
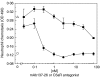
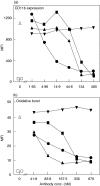
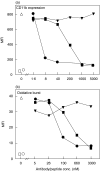
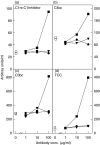
Complement C5a is aetiologically linked to inflammatory tissue damage in conditions like septicaemia, immune complex diseases and ischaemia-reperfusion injury. We here describe a monoclonal antibody (mAb), 137-26, that binds to the C5a moiety of human C5 and neutralizes the effects of C5a without interfering with C5 cleavage and the subsequent formation of lytic C5b-9 complex. Mouse anti-human C5 mAbs were generated and the reactivity with C5 and C5a was detected by ELISA and surface plasmon resonance. The inhibition of C5a binding to C5a receptor was studied using a radioligand binding assay. The effects of the antibody on C5a functions were examined using isolated neutrophils and a novel human whole blood model of inflammation. Haemolytic assays were used to study the effect on complement-mediated lysis. mAb 137-26 reacted with both solid- and solution-phase C5 and C5a in a dose-dependent manner with high affinity. The antibody competed C5a binding to C5a receptor and inhibited C5a-mediated chemotaxis of neutrophils. Furthermore, the antibody effectively abrogated complement-dependent E. coli-induced CD11b up-regulation and oxidative burst in neutrophils of human whole blood. mAb 137-26 was more potent than a C5a receptor antagonist and a previously described anti-C5a antibody. mAb 137-26 did not inhibit complement-mediated lysis, nor did it activate complement itself. Together, mAb 137-26 binds both the C5a moiety of native C5 and free C5a, thereby effectively neutralizing the biological effects of C5a. The antibody may have therapeutic potential in inflammatory diseases where C5a inhibition combined with an operative lytic pathway of C5b-9 is particularly desired.
Figures
Similar articles
-
Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis.Blood. 2003 Nov 15;102(10):3702-10. doi: 10.1182/blood-2003-03-0703. Epub 2003 Jul 24. Blood. 2003. PMID: 12881318
-
Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv.Mol Immunol. 1996 Dec;33(17-18):1389-401. doi: 10.1016/s0161-5890(96)00078-8. Mol Immunol. 1996. PMID: 9171898
-
Eculizumab-C5 complexes express a C5a neoepitope in vivo: Consequences for interpretation of patient complement analyses.Mol Immunol. 2017 Sep;89:111-114. doi: 10.1016/j.molimm.2017.05.021. Epub 2017 Jun 10. Mol Immunol. 2017. PMID: 28610663
-
Inhibiting the C5-C5a receptor axis.Mol Immunol. 2011 Aug;48(14):1631-42. doi: 10.1016/j.molimm.2011.04.014. Epub 2011 May 6. Mol Immunol. 2011. PMID: 21549429 Review.
-
C5 neoepitopes appearing during activation.Complement Inflamm. 1989;6(3):219-22. doi: 10.1159/000463095. Complement Inflamm. 1989. PMID: 2472923 Review.
Cited by
-
Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells.Cells. 2024 Aug 9;13(16):1329. doi: 10.3390/cells13161329. Cells. 2024. PMID: 39195219 Free PMC article.
-
Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies.Neuroradiology. 2007 Feb;49(2):93-102. doi: 10.1007/s00234-006-0183-z. Epub 2006 Dec 20. Neuroradiology. 2007. PMID: 17177065 Free PMC article. Review.
-
A Conformational Change of Complement C5 Is Required for Thrombin-Mediated Cleavage, Revealed by a Novel Ex Vivo Human Whole Blood Model Preserving Full Thrombin Activity.J Immunol. 2021 Sep 15;207(6):1641-1651. doi: 10.4049/jimmunol.2001471. Epub 2021 Aug 11. J Immunol. 2021. PMID: 34380648 Free PMC article.
-
Structure and characterization of a high affinity C5a monoclonal antibody that blocks binding to C5aR1 and C5aR2 receptors.MAbs. 2018 Jan;10(1):104-117. doi: 10.1080/19420862.2017.1384892. Epub 2017 Oct 24. MAbs. 2018. PMID: 28952876 Free PMC article.
-
Metallothionein mediates leukocyte chemotaxis.BMC Immunol. 2005 Sep 15;6:21. doi: 10.1186/1471-2172-6-21. BMC Immunol. 2005. PMID: 16164753 Free PMC article.
References
-
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. - PubMed
-
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. - PubMed
-
- Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–4. - PubMed
-
- Riou P. TP-10 (Avant Immunotherapeutics) Curr Opin Invest Drugs. 2001;2:364–71. - PubMed
-
- Kaplan M. Eculizumab (Alexion) Curr Opin Invest Drugs. 2002;3:1017–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

