Prospective audit of adverse reactions occurring in 459 primary antibody-deficient patients receiving intravenous immunoglobulin
- PMID: 12869031
- PMCID: PMC1808773
- DOI: 10.1046/j.1365-2249.2003.02199.x
Prospective audit of adverse reactions occurring in 459 primary antibody-deficient patients receiving intravenous immunoglobulin
Abstract
Intravenous immunoglobulin (IVIG) is used as the standard replacement therapy for patients with primary antibody deficiencies. A previous study of adverse reactions in patients self-infusing at home over 1 year showed an overall reaction rate of 0.7%. A larger prospective study is reported here, involving a greater number of immunology centres and including children and adults who received infusions from medical or nursing staff as well as those self-infusing. Four hundred and fifty-nine patients were entered into this study and 13 508 infusions were given. The study showed that no severe reactions occurred and the reaction rate was low at 0.8%. This figure could have been lower, 0.5%, if predisposing factors responsible for some reactions had been considered before infusion. In conclusion, the study shows the importance of ongoing training for patients and staff to recognize the predisposing factors to prevent avoidable reactions. Because none of these reactions were graded as severe, the present guidance to prescribe self-injectable adrenaline for patients infusing outside hospital should be reviewed.
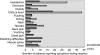
Figures
References
-
- Brennan VM, Cochrane S, Fletcher C, Hendy D, Powell P. Surveillance of adverse reactions in patients self-infusing intravenous immunoglobulin at home. J Clin Immunol. 1995;15:116–9. - PubMed
-
- Misbah SA, Chapel HM. Adverse effects of intravenous immunoglobulin. Drug Safety. 1993;9:254–62. - PubMed
-
- Bjorkander J, Hammarstrom L, Smith CIE, Buckley R, Cunningham-Rundles C, Hanson LA. Successful immunoglobulin prophylaxis in patients with antibody deficiency syndromes in the presence of anti-IgA. J Clin Immunol. 1987;7:8–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical