Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses
- PMID: 12869693
- PMCID: PMC170940
- DOI: 10.1073/pnas.1233578100
Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses
Abstract
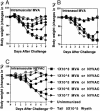
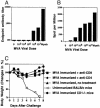
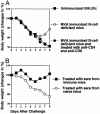
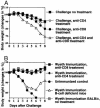
The concern about bioterrorism with smallpox has raised the possibility of widespread vaccination, but the greater prevalence of immunocompromised individuals today requires a safer vaccine, and the mechanisms of protection are not well understood. Here we show that, at sufficient doses, the protection provided by both modified vaccinia Ankara and NYVAC replication-deficient vaccinia viruses, safe in immunocompromised animals, was equivalent to that of the licensed Wyeth vaccine strain against a pathogenic vaccinia virus intranasal challenge of mice. A similar variety and pattern of immune responses were involved in protection induced by modified vaccinia Ankara and Wyeth viruses. For both, antibody was essential to protect against disease, whereas neither effector CD4+ nor CD8+ T cells were necessary or sufficient. However, in the absence of antibody, T cells were necessary and sufficient for survival and recovery. Also, T cells played a greater role in control of sublethal infection in unimmunized animals. These properties, shared with the existing smallpox vaccine, provide a basis for further evaluation of these replication-deficient vaccinia viruses as safer vaccines against smallpox or against complications from vaccinia virus.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

