Ribosomal localization of translation initiation factor IF2
- PMID: 12869707
- PMCID: PMC1370462
- DOI: 10.1261/rna.2116303
Ribosomal localization of translation initiation factor IF2
Abstract
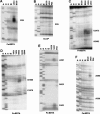
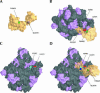
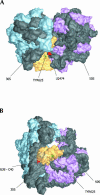
Bacterial translation initiation factor IF2 is a GTP-binding protein that catalyzes binding of initiator fMet-tRNA in the ribosomal P site. The topographical localization of IF2 on the ribosomal subunits, a prerequisite for understanding the mechanism of initiation complex formation, has remained elusive. Here, we present a model for the positioning of IF2 in the 70S initiation complex as determined by cleavage of rRNA by the chemical nucleases Cu(II):1,10-orthophenanthroline and Fe(II):EDTA tethered to cysteine residues introduced into IF2. Two specific amino acids in the GII domain of IF2 are in proximity to helices H3, H4, H17, and H18 of 16S rRNA. Furthermore, the junction of the C-1 and C-2 domains is in proximity to H89 and the thiostrepton region of 23S rRNA. The docking is further constrained by the requisite proximity of the C-2 domain with P-site-bound tRNA and by the conserved GI domain of the IF2 with the large subunit's factor-binding center. Comparison of our present findings with previous data further suggests that the IF2 orientation on the 30S subunit changes during the transition from the 30S to 70S initiation complex.
Figures



References
-
- Abel, K., Yoder, M.D., Hilgenfeld, R., and Jurnak, F. 1996. An α to β conformational switch in EF-Tu. Structure 4: 1153–1159. - PubMed
-
- Agrawal, R.K., Heagle, A.B., Penczek, P., Grassucci, R.A., and Frank, J. 1999. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat. Struct. Biol. 6: 643–647. - PubMed
-
- al-Karadaghi, S., Aevarsson, A., Garber, M., Zheltonosova, J., and Liljas, A. 1996. The structure of elongation factor G in complex with GDP: Conformational flexibility and nucleotide exchange. Structure 4: 555–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
