Human secretory immunoglobulin A may contribute to biofilm formation in the gut
- PMID: 12871226
- PMCID: PMC1782994
- DOI: 10.1046/j.1365-2567.2003.01700.x
Human secretory immunoglobulin A may contribute to biofilm formation in the gut
Abstract
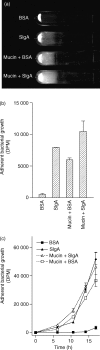
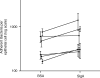
It is critical, both for the host and for the long-term benefit of the bacteria that colonize the gut, that bacterial overgrowth with subsequent bacterial translocation, which may lead to sepsis and death of the host, be avoided. Secretory IgA (sIgA) is known to be a key factor in this process, agglutinating bacteria and preventing their translocation in a process termed 'immune exclusion'. To determine whether human sIgA might facilitate the growth of normal enteric bacteria under some conditions, the growth of human enteric bacteria on cultured, fixed human epithelial cells was evaluated in the presence of sIgA or various other proteins. Human sIgA was found to facilitate biofilm formation by normal human gut flora and by Escherichia coli on cultured human epithelial cell surfaces under conditions in which non-adherent bacteria were repeatedly washed away. In addition, the presence of sIgA resulted in a 64% increase in adherence of E. coli to live cultured epithelial cells over a 45-min period. Mucin, another defence factor thought to play a key role in immune exclusion, was found to facilitate biofilm formation by E. coli. Our findings suggest that sIgA may contribute to biofilm formation in the gut.
Figures

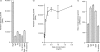


References
-
- Biancone L, Monteleone I, Del Vecchio Blanco G, Vavassori P, Pallone F. Resident bacterial flora and immune system. Digest Liver Dis. 2002;34:S37–43. - PubMed
-
- Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002;56:S60–4. - PubMed
-
- Williams RC, Gibbons RJ. Inhibition of bacterial adherence by secretory immunoglobulin A. a mechanism of antigen disposal. Science. 1972;177:697–9. - PubMed
-
- Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

