Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures
- PMID: 12871597
- PMCID: PMC166161
- DOI: 10.1186/1475-2859-2-6
Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures
Abstract
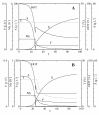
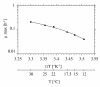
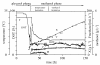
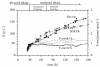
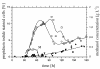
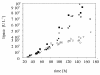
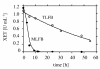
BACKGROUND: A temperature limited fed-batch (TLFB) technique is described and used for Pichia pastoris Mut+ strain cultures and compared with the traditional methanol limited fed-batch (MLFB) technique. A recombinant fusion protein composed of a cellulose-binding module (CBM) from Neocallimastix patriciarum cellulase 6A and lipase B from Candida antarctica (CALB), was produced and secreted by this strain. RESULTS: A protein concentration of about 1 g L-1 was produced in the MLFB process. However, this product was considerably degraded by protease(s). By applying the TLFB process, the yield was increased to 2 g L-1 full-length product and no proteolytic degradation was observed. Flow cytometry analysis showed that the percentage of dead cells increased rapidly during the initial methanol feed phase in the MLFB process and reached a maximum of about 12% after about 40-70 hours of methanol feeding. In the TLFB process, cell death rate was low and constant and reached 4% dead cells at the end of cultivation (about 150 hours methanol feeding time). The lower cell death rate in the TLFB correlated with a lower protease activity in the culture supernatant. The specific alcohol oxidase (AOX) activity in the TLFB process was 3.5 times higher than in the MLFB process. CONCLUSION: Three mechanisms that may contribute to the much higher accumulation of product in the TLFB process are: 1) reduced proteolysis due to lower temperature, 2) reduced proteolysis due to lower cell death and protease release to the medium, 3) increased synthesis rate due to higher AOX activity.
Figures


References
-
- Cregg MJ, Cereghino LJ, Shi J, Higgins RD. Recombinant Protein Expression in Pichia pastoris. Mol Biotechnol. 2000;16:23–52. - PubMed
-
- Cregg MJ, Vedvick ST, Raschke CW. Recent Advances in the Expression of Foreign Genes in Pichia pastoris. Bio/Technology. 1993;11:905–910. - PubMed
-
- Romanos M. Advances in the use of Pichia pastoris for high level gene expression. Curr Opin Biotechnol. 1995;6:527–533.
-
- Jahic M, Gustavsson M, Jansen AK, Martinelle M, Enfors S-O. Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch process. J Biotechnol. 2003;102:45–53. - PubMed
-
- Chen Y, Cino J, Hart G, Freedman D, White C, Komives EA. High protein expression in fermentation of recombinant Pichia pastoris by a fed-batch process. Process Biochem. 1997;32:107–111.
LinkOut - more resources
Full Text Sources
Other Literature Sources

