Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy
- PMID: 12872001
- PMCID: PMC187908
- DOI: 10.1073/pnas.1533448100
Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy
Abstract
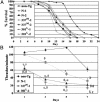
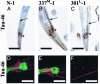
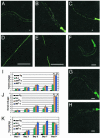
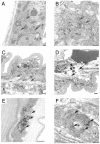
Frontotemporal dementia with parkinsonism chromosome 17 type (FTDP-17) is caused by mutations in MAPT, the gene encoding tau. FTDP-17 begins with executive function deficits and other abnormal behaviors, which progress to dementia. Neurodegenerative changes include accumulation of aggregated tau as neuronal and glial fibrillary tangles. Aggregated tau is seen in numerous other neurodegenerative diseases, including Alzheimer's disease (AD). We expressed normal and FTDP-17 mutant human tau (mutations P301L and V337M) in Caenorhabditis elegans to model tauopathy disorders. Tau pan-neuronal expression caused progressive uncoordinated locomotion (Unc), characteristic of nervous system defects in worms. Subsequently, insoluble tau accumulates and both soluble and insoluble tau is phosphorylated at many of the sites hyperphosphorylated in FTDP-17, AD, and other tauopathies. Substantial neurodegeneration, seen as bulges and gaps in nerve cords followed by loss of neurons, occurs after insoluble tau begins to accumulate. Axons show vacuoles, membranous infoldings, and whorls with associated amorphous tau accumulations and abnormal tau-positive aggregates. FTDP-17 mutation lines had a more severe Unc phenotype, accumulated more insoluble tau at a younger age, were more resistant to cholinergic inhibitors, and had more severe axonal degeneration when compared with lines expressing normal tau. The Unc phenotype is caused by a presynaptic defect. Postsynaptic transmission is intact. This transgenic model will enable mechanistic dissection of tau-induced neurodegeneration and identification of genes and compounds that inhibit pathological tau formation.
Figures


Comment in
-
Neurodegenerative tauopathy in the worm.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9653-5. doi: 10.1073/pnas.1834191100. Epub 2003 Aug 11. Proc Natl Acad Sci U S A. 2003. PMID: 12913116 Free PMC article. No abstract available.
References
-
- Hutton, M., Lendon, C. L., Rizzu, P., Baker, M., Froelich, S., Houlden, H., Pickering-Brown, S., Chakraverty, S., Isaacs, A., Grover, A., et al. (1998) Nature 393, 702–705. - PubMed
-
- Poorkaj, P., Bird, T. D., Wijsman, E., Nemens, E., Garruto, R. M., Anderson, L., Andreadis, A., Wiederholt, W. C., Raskind, M. & Schellenberg, C. D. (1998) Ann. Neurol. 43, 815–825. - PubMed
-
- Hong, M., Zhukareva, V., Vogelsberg-Ragaglia, V., Wszolek, Z., Reed, L., Miller, B. I., Geschwind, D. H., Bird, T. D., McKeel, D. Goate, A., et al. (1998) Science 282, 1914–1917. - PubMed
-
- Goedert, M., Jakes, R. & Crowther, R. A. (1999) FEBS Lett. 450, 306–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

