Episodic phrenic-inhibitory vagus nerve stimulation paradoxically induces phrenic long-term facilitation in rats
- PMID: 12872010
- PMCID: PMC2343284
- DOI: 10.1113/jphysiol.2003.048157
Episodic phrenic-inhibitory vagus nerve stimulation paradoxically induces phrenic long-term facilitation in rats
Abstract
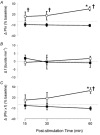
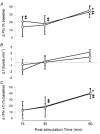
All respiratory long-term facilitation (LTF) is induced by inspiratory-excitatory stimulation, suggesting that LTF needs inspiratory augmentation and is the result of a Hebbian mechanism (coincident pre- and post-synaptic activity strengthens synapses). The present study examined the long-term effects of episodic inspiratory-inhibitory vagus nerve stimulation (VNS) on phrenic nerve activity. We hypothesized that episodic VNS would induce phrenic long-term depression. The results are compared with those obtained following serotonin receptor antagonism or episodic carotid sinus nerve stimulation (CSNS). Integrated phrenic neurograms were measured before, during and after three episodes of 5 min VNS (50 Hz, 0.1 ms), each separated by a 5 min interval, at a low (approximately 50 microA), medium (approximately 200 microA) or high (approximately 500 microA) stimulus intensity in anaesthetized, vagotomized, neuromuscularly blocked and artificially ventilated rats. Medium- and high-intensity VNS eliminated rhythmic phrenic activity during VNS, while low-intensity VNS only reduced phrenic burst frequency. At 60 min post-VNS, phrenic amplitude was higher than baseline (35 +/- 5% above baseline, mean +/- S.E.M., P < 0.05) in the high-intensity group but not in the low- (-4 +/- 4%) or medium-intensity groups (-10 +/- 15%), or in the high-intensity with methysergide group (4 mg kg(-1), i.p.) (-11 +/- 5%). These data, which are inconsistent with our hypothesis, indicate that phrenic-inhibitory VNS induces a serotonin-dependent phrenic LTF similar to that induced by phrenic-excitatory CSNS (33 +/- 7%) and may require activation of high-threshold afferent fibres. These data also suggest that the synapses on phrenic motoneurons do not use the Hebbian mechanism in this LTF, as these motoneurons were suppressed during VNS.
Figures


Similar articles
-
Serotonin receptor subtypes involved in vagus nerve stimulation-induced phrenic long-term facilitation in rats.Neurosci Lett. 2004 Jun 10;363(2):108-11. doi: 10.1016/j.neulet.2004.03.067. Neurosci Lett. 2004. PMID: 15172095
-
Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats.J Physiol. 2005 Sep 1;567(Pt 2):599-611. doi: 10.1113/jphysiol.2005.087650. Epub 2005 Jun 2. J Physiol. 2005. PMID: 15932891 Free PMC article.
-
Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats.J Physiol. 1994 Jun 15;477 ( Pt 3)(Pt 3):469-79. doi: 10.1113/jphysiol.1994.sp020208. J Physiol. 1994. PMID: 7932235 Free PMC article.
-
NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation.J Physiol. 2009 May 1;587(Pt 9):1931-42. doi: 10.1113/jphysiol.2008.165597. Epub 2009 Feb 23. J Physiol. 2009. PMID: 19237427 Free PMC article. Review.
-
Long term facilitation of phrenic motor output.Respir Physiol. 2000 Jul;121(2-3):135-46. doi: 10.1016/s0034-5687(00)00124-9. Respir Physiol. 2000. PMID: 10963770 Review.
Cited by
-
Urethane inhibits genioglossal long-term facilitation in un-paralyzed anesthetized rats.Neurosci Lett. 2010 Jun 25;477(3):124-8. doi: 10.1016/j.neulet.2010.04.047. Epub 2010 Apr 28. Neurosci Lett. 2010. PMID: 20433898 Free PMC article.
-
Experimental protocols and preparations to study respiratory long term facilitation.Respir Physiol Neurobiol. 2011 Apr 30;176(1-2):1-11. doi: 10.1016/j.resp.2011.01.007. Epub 2011 Feb 1. Respir Physiol Neurobiol. 2011. PMID: 21292044 Free PMC article. Review.
-
Identification of a novel form of noradrenergic-dependent respiratory motor plasticity triggered by vagal feedback.J Neurosci. 2010 Dec 15;30(50):16886-95. doi: 10.1523/JNEUROSCI.3394-10.2010. J Neurosci. 2010. PMID: 21159960 Free PMC article.
-
Reduced respiratory neural activity elicits phrenic motor facilitation.Respir Physiol Neurobiol. 2011 Mar 15;175(3):303-9. doi: 10.1016/j.resp.2010.12.005. Epub 2010 Dec 15. Respir Physiol Neurobiol. 2011. PMID: 21167322 Free PMC article.
-
Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation.J Neurosci. 2012 Nov 14;32(46):16510-20. doi: 10.1523/JNEUROSCI.2631-12.2012. J Neurosci. 2012. PMID: 23152633 Free PMC article.
References
-
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. - PubMed
-
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory. Nat Rev Neurosci. 2000;1:11–20. - PubMed
-
- Bavis RW, Mitchell GS. Plasticity in respiratory motor control: selected contribution: intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol. 2003;94:399–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

