Altered fracture repair in the absence of MMP9
- PMID: 12874132
- PMCID: PMC2778064
- DOI: 10.1242/dev.00559
Altered fracture repair in the absence of MMP9
Abstract
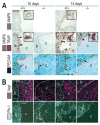
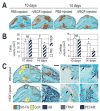
The regeneration of adult skeletal tissues requires the timely recruitment of skeletal progenitor cells to an injury site, the differentiation of these cells into bone or cartilage, and the re-establishment of a vascular network to maintain cell viability. Disturbances in any of these cellular events can have a detrimental effect on the process of skeletal repair. Although fracture repair has been compared with fetal skeletal development, the extent to which the reparative process actually recapitulates the fetal program remains uncertain. Here, we provide the first genetic evidence that matrix metalloproteinase 9 (MMP9) regulates crucial events during adult fracture repair. We demonstrate that MMP9 mediates vascular invasion of the hypertrophic cartilage callus, and that Mmp9(-/-) mice have non-unions and delayed unions of their fractures caused by persistent cartilage at the injury site. This MMP9- dependent delay in skeletal healing is not due to a lack of vascular endothelial growth factor (VEGF) or VEGF receptor expression, but may instead be due to the lack of VEGF bioavailability in the mutant because recombinant VEGF can rescue Mmp9(-/-) non-unions. We also found that Mmp9(-/-) mice generate a large cartilage callus even when fractured bones are stabilized, which implicates MMP9 in the regulation of chondrogenic and osteogenic cell differentiation during early stages of repair. In conclusion, the resemblance between Mmp9(-/-) fetal skeletal defects and those that emerge during Mmp9(-/-) adult repair offer the strongest evidence to date that similar mechanisms are employed to achieve bone formation, regardless of age.
Figures




References
-
- Albrecht U, Eichele G, Helms JA, Lin H. Visualization of gene expression patterns by in situ hybridization. Boca Raton, FL: CRC Press; 1997.
-
- Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. - PubMed
-
- Banovac K, Renfree K, Makowski AL, Latta LL, Altman RD. Fracture healing and mast cells. J Orthop Trauma. 1995;9:482–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

