Paired activating and inhibitory immunoglobulin-like receptors, MAIR-I and MAIR-II, regulate mast cell and macrophage activation
- PMID: 12874256
- PMCID: PMC2194075
- DOI: 10.1084/jem.20021825
Paired activating and inhibitory immunoglobulin-like receptors, MAIR-I and MAIR-II, regulate mast cell and macrophage activation
Abstract
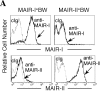
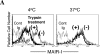
Immune responses are regulated by opposing positive and negative signals triggered by the interaction of activating and inhibitory cell surface receptors with their ligands. Here, we describe novel paired activating and inhibitory immunoglobulin-like receptors, designated myeloid-associated immunoglobulin-like receptor (MAIR) I and MAIR-II, whose extracellular domains are highly conserved by each other. MAIR-I, expressed on the majority of myeloid cells, including macrophages, granulocytes, mast cells, and dendritic cells, contains the tyrosine-based sorting motif and the immunoreceptor tyrosine-based inhibitory motif-like sequences in the cytoplasmic domain and mediates endocytosis of the receptor and inhibition of IgE-mediated degranulation from mast cells. On the other hand, MAIR-II, expressed on subsets of peritoneal macrophages and B cells, associates with the immunoreceptor tyrosine-based activation motif-bearing adaptor DAP12 and stimulates proinflammatory cytokines and chemokine secretions from macrophages. Thus, MAIR-I and MAIR-II play important regulatory roles in cell signaling and immune responses.
Figures

















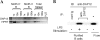
References
-
- Ravetch, J.V., and L.L. Lanier. 2000. Immune inhibitory receptors. Science. 290:84–89. - PubMed
-
- Lanier, L.L. 2001. Face off–the interplay between activating and inhibitory immune receptors. Curr. Opin. Immunol. 13:326–331. - PubMed
-
- Malbec, O., D.C. Fong, M. Turner, V.L. Tybulewicz, J.C. Cambier, W.H. Fridman, and M. Daeron. 1998. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J. Immunol. 160:1647–1658. - PubMed
-
- Takai, T., and M. Ono. 2001. Activating and inhibitory nature of the murine paired immunoglobulin-like receptor family. Immunol. Rev. 181:215–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

