XIAP-mediated caspase inhibition in Hodgkin's lymphoma-derived B cells
- PMID: 12874265
- PMCID: PMC2194071
- DOI: 10.1084/jem.20021279
XIAP-mediated caspase inhibition in Hodgkin's lymphoma-derived B cells
Abstract
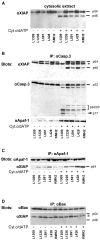
The malignant Hodgkin and Reed-Sternberg cells of Hodgkin's lymphoma (HL) and HL-derived B cell lines were previously shown to be resistant to different apoptotic stimuli. We show here that cytochrome c fails to stimulate caspases-9 and -3 activation in cytosolic extracts of HL-derived B cells, which is due to high level expression of X-linked inhibitor of apoptosis (XIAP). Coimmunoprecipitation studies revealed that XIAP, apoptosis protease-activating factor-1, and caspase-3 are complexed in HL-derived B cell lysates. Even after stimulation with exogenous cytochrome c and dATP, XIAP impairs the proteolytic processing and activation of caspase-3. In cytosolic extracts, inhibition of XIAP by the second mitochondria-derived activator of caspases (Smac)/DIABLO, or immunodepletion of XIAP restores cytochrome c-triggered processing and activation of caspase-3. Smac or a Smac-derived agonistic peptide also sensitized intact HL-derived B cells for the apoptotic action of staurosporine. Finally, Hodgkin and Reed-Sternberg cells of primary tumor HL tissues also constitutively and abundantly express XIAP. The results of this paper suggest that high level XIAP expression is a hallmark of HL, which may play a crucial role in resistance to apoptosis.
Figures







References
-
- Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 102:33–42. - PubMed
-
- Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, E.S. Alnemri, and X.D. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

