Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis
- PMID: 12874303
- PMCID: PMC166010
- DOI: 10.1128/IAI.71.8.4278-4288.2003
Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis
Abstract
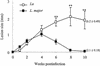
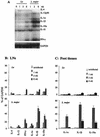
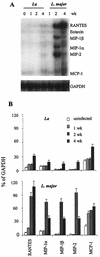
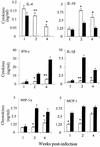
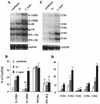
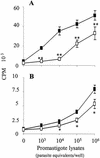
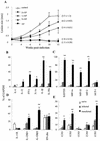
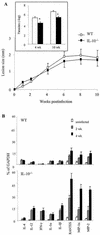
Infection of mice with Leishmania major results in disease progression or resolution, largely depending on the genetic backgrounds of the mouse strains. Infection with Leishmania amazonensis, on the other hand, causes progressive cutaneous lesions in most inbred strains of mice. We hypothesized that deficient activation of early immune responses contributes to the pathogenesis in L. amazonensis-infected mice. To distinguish early molecular events that determine the outcome of Leishmania infections, we examined cytokine gene expression in C57BL/6 mice infected with either L. amazonensis or L. major (a healing model). After 2 to 4 weeks, L. amazonensis-infected mice had significantly delayed and depressed expression of inflammatory cytokines (interleukin-12 [IL-12], gamma interferon, IL-1 alpha, IL-1 beta), CC chemokines (CC chemokine ligand 3 [CCL3]/macrophage inflammatory protein 1 alpha [MIP-1 alpha], CCL4/MIP-1 beta, CCL5/RANTES, MIP-2), and chemokine receptors (CCR1, CCR2, CCR5) in foot tissues and draining lymph nodes compared to the expression in L. major-infected controls. These findings correlated with defective T-cell responsiveness to parasite stimulation in vivo and in vitro. Adoptive transfer of L. amazonensis-specific Th1 cells prior to infection overcame the immune defects of the animals, leading to complete control of the disease. Studies with gene knockout mice suggested that IL-10, but not IL-4, contributed partially to compromised immunity in L. amazonensis-infected hosts. The data suggest that there is impairment in multiple immune functions at early stages of infection with L. amazonensis parasites and provide a compelling rationale to explore immune augmentation as an intervention in American cutaneous leishmaniasis.
Figures
Similar articles
-
IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection.J Immunol. 2000 Jul 1;165(1):364-72. doi: 10.4049/jimmunol.165.1.364. J Immunol. 2000. PMID: 10861073
-
Early infection with Leishmania major restrains pathogenic response to Leishmania amazonensis and parasite growth.Acta Trop. 2008 Apr;106(1):27-38. doi: 10.1016/j.actatropica.2007.12.012. Epub 2008 Jan 15. Acta Trop. 2008. PMID: 18313021
-
Vaccine-induced protection against Leishmania amazonensis is obtained in the absence of IL-12/23p40.Immunol Lett. 2006 May 15;105(1):38-47. doi: 10.1016/j.imlet.2005.12.002. Epub 2006 Jan 13. Immunol Lett. 2006. PMID: 16466810
-
The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis.J Immunol. 2003 Oct 1;171(7):3705-10. doi: 10.4049/jimmunol.171.7.3705. J Immunol. 2003. PMID: 14500669
-
Role of host genetics and cytokines in Leishmania infection.Cytokine. 2021 Nov;147:155244. doi: 10.1016/j.cyto.2020.155244. Epub 2020 Oct 12. Cytokine. 2021. PMID: 33059974 Review.
Cited by
-
Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages.Infect Immun. 2007 Feb;75(2):653-65. doi: 10.1128/IAI.01314-06. Epub 2006 Nov 6. Infect Immun. 2007. PMID: 17088347 Free PMC article.
-
Role of interleukin-1beta in activating the CD11c(high) CD45RB- dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo.Infect Immun. 2007 Oct;75(10):5018-26. doi: 10.1128/IAI.00499-07. Epub 2007 Aug 6. Infect Immun. 2007. PMID: 17682041 Free PMC article.
-
Propolis reduces Leishmania amazonensis-induced inflammation in the liver of BALB/c mice.Parasitol Res. 2016 Apr;115(4):1557-66. doi: 10.1007/s00436-015-4890-4. Epub 2015 Dec 28. Parasitol Res. 2016. PMID: 26711452
-
Modeling Immune Response to Leishmania Species Indicates Adenosine As an Important Inhibitor of Th-Cell Activation.Front Cell Infect Microbiol. 2017 Jul 20;7:309. doi: 10.3389/fcimb.2017.00309. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28775959 Free PMC article.
-
Combined therapy with adipose tissue-derived mesenchymal stromal cells and meglumine antimoniate controls lesion development and parasite load in murine cutaneous leishmaniasis caused by Leishmania amazonensis.Stem Cell Res Ther. 2020 Aug 31;11(1):374. doi: 10.1186/s13287-020-01889-z. Stem Cell Res Ther. 2020. PMID: 32867857 Free PMC article.
References
-
- Almeida, R. P., M. Barral-Netto, A. M. De Jesus, L. A. De Freitas, E. M. Carvalho, and A. Barral. 1996. Biological behavior of Leishmania amazonensis isolated from humans with cutaneous, mucosal, or visceral leishmaniasis in BALB/C mice. Am. J. Trop. Med. Hyg. 54:178-184. - PubMed
-
- Arend, W. P. 2002. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 13:323-340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

