Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols
- PMID: 12874310
- PMCID: PMC165995
- DOI: 10.1128/IAI.71.8.4333-4340.2003
Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols
Abstract
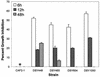
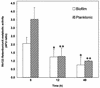
Candida albicans biofilms are formed through three distinct developmental phases and are associated with high fluconazole (FLU) resistance. In the present study, we used a set of isogenic Candida strains lacking one or more of the drug efflux pumps Cdr1p, Cdr2p, and Mdr1p to determine their role in FLU resistance of biofilms. Additionally, variation in sterol profile as a possible mechanism of drug resistance was investigated. Our results indicate that parent and mutant strains formed similar biofilms. However, biofilms formed by double and triple mutants were more susceptible to FLU at 6 h (MIC = 64 and 16 microg/ml, respectively) than the wild-type strain (MIC > 256 microg/ml). At later time points (12 and 48 h), all the strains became resistant to this azole (MIC > or = 256 microg/ml), indicating lack of involvement of efflux pumps in resistance at late stages of biofilm formation. Northern blot analyses revealed that Candida biofilms expressed CDR and MDR1 genes in all the developmental phases, while planktonic cells expressed these genes only at the 12- and 48-h time points. Functionality of efflux pumps was assayed by rhodamine (Rh123) efflux assays, which revealed significant differences in Rh123 retention between biofilm and planktonic cells at the early phase (P = 0.0006) but not at later stages (12 and 48 h). Sterol analyses showed that ergosterol levels were significantly decreased (P < 0.001) at intermediate and mature phases, compared to those in early-phase biofilms. These studies suggest that multicomponent, phase-specific mechanisms are operative in antifungal resistance of fungal biofilms.
Figures


Similar articles
-
[Investigation of the expression levels of efflux pumps in fluconazole-resistant Candida albicans isolates].Mikrobiyol Bul. 2014 Apr;48(2):325-34. doi: 10.5578/mb.7213. Mikrobiyol Bul. 2014. PMID: 24819270 Turkish.
-
Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms.J Antimicrob Chemother. 2002 Jun;49(6):973-80. doi: 10.1093/jac/dkf049. J Antimicrob Chemother. 2002. PMID: 12039889
-
ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates.Antimicrob Agents Chemother. 2008 Nov;52(11):3851-62. doi: 10.1128/AAC.00463-08. Epub 2008 Aug 18. Antimicrob Agents Chemother. 2008. PMID: 18710914 Free PMC article.
-
[Advances in the study of Candida albicans gene mutation on azole drug resistance].Yao Xue Xue Bao. 2010 Jul;45(7):821-6. Yao Xue Xue Bao. 2010. PMID: 20931777 Review. Chinese.
-
Update on the structure and function of Candida albicans drug efflux protein, Cdr1.Fungal Genet Biol. 2024 Dec;175:103938. doi: 10.1016/j.fgb.2024.103938. Epub 2024 Oct 30. Fungal Genet Biol. 2024. PMID: 39486613 Review.
Cited by
-
Thidiazuron, a phenyl-urea cytokinin, inhibits ergosterol synthesis and attenuates biofilm formation of Candida albicans.World J Microbiol Biotechnol. 2022 Sep 17;38(12):224. doi: 10.1007/s11274-022-03410-5. World J Microbiol Biotechnol. 2022. PMID: 36114903
-
Community participation in biofilm matrix assembly and function.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):4092-7. doi: 10.1073/pnas.1421437112. Epub 2015 Mar 13. Proc Natl Acad Sci U S A. 2015. PMID: 25770218 Free PMC article.
-
Fungal biofilm resistance.Int J Microbiol. 2012;2012:528521. doi: 10.1155/2012/528521. Epub 2012 Feb 8. Int J Microbiol. 2012. PMID: 22518145 Free PMC article.
-
The calcineruin inhibitor cyclosporine a synergistically enhances the susceptibility of Candida albicans biofilms to fluconazole by multiple mechanisms.BMC Microbiol. 2016 Jun 18;16(1):113. doi: 10.1186/s12866-016-0728-1. BMC Microbiol. 2016. PMID: 27316338 Free PMC article.
-
A contact-activated kinase signals Candida albicans invasive growth and biofilm development.Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5576-81. doi: 10.1073/pnas.0407097102. Epub 2005 Mar 30. Proc Natl Acad Sci U S A. 2005. PMID: 15800048 Free PMC article.
References
-
- Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

