Structure of the TDP-epi-vancosaminyltransferase GtfA from the chloroeremomycin biosynthetic pathway
- PMID: 12874381
- PMCID: PMC170902
- DOI: 10.1073/pnas.1233577100
Structure of the TDP-epi-vancosaminyltransferase GtfA from the chloroeremomycin biosynthetic pathway
Abstract
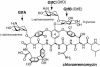
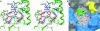
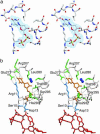
During the biosynthesis of the vancomycin-class antibiotic chloroeremomycin, TDP-epi-vancosaminyltransferase GtfA catalyzes the attachment of 4-epi-vancosamine from a TDP donor to the beta-OHTyr-6 of the aglycone cosubstrate. Glycosyltransferases from this pathway are potential tools for the combinatorial design of new antibiotics that are effective against vancomycin-resistant bacterial strains. These enzymes are members of the GT-B glycosyltransferase superfamily, which share a homologous bidomain topology. We present the 2.8-A crystal structures of GtfA complexes with vancomycin and the natural monoglycosylated peptide substrate, representing the first direct observation of acceptor substrate binding among closely related glycosyltransferases. The acceptor substrates bind to the N-terminal domain such that the aglycone substrate's reactive hydroxyl group hydrogen bonds to the side chains of Ser-10 and Asp-13, thus identifying these as residues of potential catalytic importance. As well as an open form of the enzyme, the crystal structures have revealed a closed form in which a TDP ligand is bound at a donor substrate site in the interdomain cleft, thereby illustrating not only binding interactions, but the conformational changes in the enzyme that accompany substrate binding.
Figures


References
-
- Solenberg, P. J., Matsushima, P., Stack, D. R., Wilkie, S. C., Thompson, R. C. & Baltz, R. H. (1997) Chem. Biol. 4, 195–202. - PubMed
-
- Rodriguez, M., Snyder, N., Zweifel, M., Wilkie, S. C., Stack, D. R., Cooper, R. D. G., Nicas, T., Mullen, D., Butler, T. & Thompson, R. C. (1998) J. Antibiot. 51, 560–569. - PubMed
-
- Ge, M., Chen, Z., Onishi, H. R., Kohler, J., Silver, L. L., Kerns, R., Fukuzawa, S., Thompson, C. & Kahne, D. (1999) Science 284, 507–511. - PubMed
-
- Losey, H. C., Peczuh, M. W., Chen, Z., Eggert, U. S., Dong, S. D., Pelczer, I., Kahne, D. & Walsh, C. T. (2001) Biochemistry 40, 4745–4755. - PubMed
-
- Losey, H. C., Jiang, J., Biggins, J. B., Oberthur, M., Ye, X.-Y., Dong, S. D., Kahne, D., Thorson, J. S. & Walsh, C. T. (2002) Chem. Biol. 9, 1305–1314. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

