When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism
- PMID: 12874385
- PMCID: PMC187858
- DOI: 10.1073/pnas.1632156100
When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism
Abstract
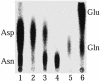
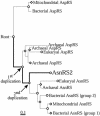
Faithful protein synthesis relies on a family of essential enzymes called aminoacyl-tRNA synthetases, assembled in a piecewise fashion. Analysis of the completed archaeal genomes reveals that all archaea that possess asparaginyl-tRNA synthetase (AsnRS) also display a second ORF encoding an AsnRS truncated from its anticodon binding-domain (AsnRS2). We show herein that Pyrococcus abyssi AsnRS2, in contrast to AsnRS, does not sustain asparaginyl-tRNAAsn synthesis but is instead capable of converting aspartic acid into asparagine. Functional analysis and complementation of an Escherichia coli asparagine auxotrophic strain show that AsnRS2 constitutes the archaeal homologue of the bacterial ammonia-dependent asparagine synthetase A (AS-A), therefore named archaeal asparagine synthetase A (AS-AR). Primary sequence- and 3D-based phylogeny shows that an archaeal AspRS ancestor originated AS-AR, which was subsequently transferred into bacteria by lateral gene transfer in which it underwent structural changes producing AS-A. This study provides evidence that a contemporary aminoacyl-tRNA synthetase can be recruited to sustain amino acid metabolism.
Figures
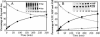

Comment in
-
tRNA synthetase paralogs: evolutionary links in the transition from tRNA-dependent amino acid biosynthesis to de novo biosynthesis.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9650-2. doi: 10.1073/pnas.1934245100. Epub 2003 Aug 11. Proc Natl Acad Sci U S A. 2003. PMID: 12913115 Free PMC article. No abstract available.
References
-
- Ibba, M., Francklyn, C. & Cusack, S., eds. (2003) The Aminoacyl-tRNA Synthetases (Landes Bioscience, Georgetown, TX), in press.
-
- Ibba, M., Becker, H. D., Stathopoulos, C., Tumbula, D. L. & Söll, D. (2000) Trends Biochem. Sci. 25, 311–316. - PubMed
-
- Curnow, A. W., Ibba, M. & Söll, D. (1996) Nature 382, 589–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

