Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas
- PMID: 12875963
- PMCID: PMC1868194
- DOI: 10.1016/S0002-9440(10)63671-1
Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas
Abstract
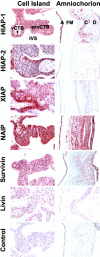
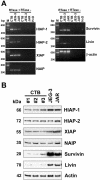
The apoptosis cascade that plays a central role in normal and pathological processes is strictly controlled, in part by newly described members of the inhibitor of apoptosis (IAP) family (HIAP-1, HIAP-2, XIAP, NAIP, Survivin, and Livin). Here, we report the expression of IAP mRNAs and proteins in early and late gestation human placentas, term cytotrophoblast cells, and two choriocarcinoma cell lines, JEG-3 and Jar. Reverse transcriptase-polymerase chain reaction identified mRNAs derived from all of the currently known IAP genes in all samples. Analysis by immunoblotting revealed that IAP proteins are present in early and late gestation human placentas and that levels of IAPs are not identical in normal and transformed trophoblast cells. Immunohistochemical experiments performed on paraformaldehyde-fixed tissue sections taken from early and late stages of pregnancy demonstrated that expression patterns differed according to cell lineage and stage of cell differentiation. The results of this study are consistent with the postulate that IAP proteins have critical roles in placental cell survival and suggest that specific apoptosis inhibitors may protect normal and transformed trophoblast cells from cell death.
Figures



References
-
- Strasser A, O’Connor L, Dixit VM: Apoptosis signaling. Annu Rev Biochem 2000, 69:217-245 - PubMed
-
- Bortner CD, Cidlowski JA: Cellular mechanisms for the repression of apoptosis. Annu Rev Pharmacol Toxicol 2002, 42:259-281 - PubMed
-
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV: The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995, 83:1243-1252 - PubMed
-
- Deveraux QL, Reed JC: IAP family proteins—suppressors of apoptosis. Genes Dev 1999, 13:239-252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

